 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Conteben |
| Other names | Thiacetazone; Thiocetazone; Thioparamizone; Benzothiozane; 4-Acetylaminobenzaldehyde thiosemicarbazone; N-[4-[(Carbamothioylhydrazinylidene) methyl]phenyl]acetamide |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.002.882 |
| Chemical and physical data | |
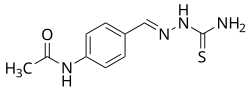
| Formula | C10H12N4OS |
| Molar mass | 236.29 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Thioacetazone (INN, BAN), also known as amithiozone (USAN), is an oral antibiotic which is used in the treatment of tuberculosis. [1] [2] [3] [4] It has fallen into almost complete disuse due to toxicity and the introduction of improved anti-tuberculosis drugs like isoniazid. [5] The drug has only weak activity against Mycobacterium tuberculosis and is only useful in preventing resistance to more powerful drugs such as isoniazid and rifampicin. It is never used on its own to treat tuberculosis; it is used in a similar way to ethambutol.
Contents
There is no advantage to using thioacetazone if the regimen used already contains ethambutol, but many countries in sub-Saharan Africa still use thioacetazone because it is extremely cheap. Use of thioacetazone is declining because it can cause severe (sometimes fatal) skin reactions in HIV positive patients. [6] [7]
The biological target of thioacetazone has proven elusive and its mechanism of action remains unknown, although it is thought to interfere with mycolic acid synthesis. [4]