This article needs additional citations for verification .(August 2013) |
 | |
| Clinical data | |
|---|---|
| Trade names | Securopen |
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.048.483 |
| Chemical and physical data | |
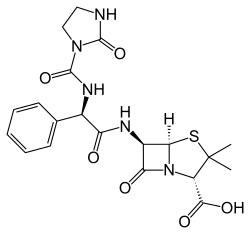
| Formula | C20H23N5O6S |
| Molar mass | 461.49 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Azlocillin is an acyl ampicillin antibiotic with an extended spectrum of activity and greater in vitro potency than the carboxy penicillins. Azlocillin is similar to mezlocillin and piperacillin. It demonstrates antibacterial activity against a broad spectrum of bacteria, including Pseudomonas aeruginosa, and, in contrast to most cephalosporins, exhibits activity against enterococci.

