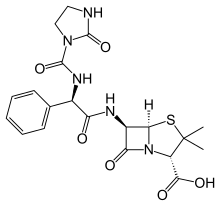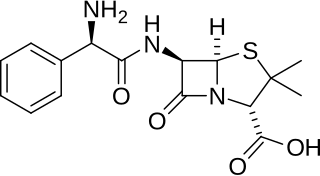
Ampicillin is an antibiotic used to prevent and treat a number of bacterial infections, such as respiratory tract infections, urinary tract infections, meningitis, salmonellosis, and endocarditis. It may also be used to prevent group B streptococcal infection in newborns. It is used by mouth, by injection into a muscle, or intravenously. Common side effects include rash, nausea, and diarrhea. It should not be used in people who are allergic to penicillin. Serious side effects may include Clostridium difficile colitis or anaphylaxis. While usable in those with kidney problems, the dose may need to be decreased. Its use during pregnancy and breastfeeding appears to be generally safe.
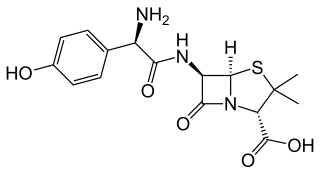
Amoxicillin is an antibiotic medication used to treat a number of bacterial infections. These include middle ear infection, strep throat, pneumonia, skin infections, and urinary tract infections among others. It is taken by mouth, or less commonly by injection.

Beta-lactamases, (β-lactamases) are enzymes produced by bacteria that provide multi-resistance to beta-lactam antibiotics such as penicillins, cephalosporins, cephamycins, monobactams and carbapenems (ertapenem), although carbapenems are relatively resistant to beta-lactamase. Beta-lactamase provides antibiotic resistance by breaking the antibiotics' structure. These antibiotics all have a common element in their molecular structure: a four-atom ring known as a beta-lactam (β-lactam) ring. Through hydrolysis, the enzyme lactamase breaks the β-lactam ring open, deactivating the molecule's antibacterial properties.

Penicillins are a group of β-lactam antibiotics originally obtained from Penicillium moulds, principally P. chrysogenum and P. rubens. Most penicillins in clinical use are synthesised by P. chrysogenum using deep tank fermentation and then purified. A number of natural penicillins have been discovered, but only two purified compounds are in clinical use: penicillin G and penicillin V. Penicillins were among the first medications to be effective against many bacterial infections caused by staphylococci and streptococci. They are still widely used today for different bacterial infections, though many types of bacteria have developed resistance following extensive use.

The cephalosporins are a class of β-lactam antibiotics originally derived from the fungus Acremonium, which was previously known as Cephalosporium.

Cefazolin, also known as cefazoline and cephazolin, is a first-generation cephalosporin antibiotic used for the treatment of a number of bacterial infections. Specifically it is used to treat cellulitis, urinary tract infections, pneumonia, endocarditis, joint infection, and biliary tract infections. It is also used to prevent group B streptococcal disease around the time of delivery and before surgery. It is typically given by injection into a muscle or vein.

Carbenicillin is a bactericidal antibiotic belonging to the carboxypenicillin subgroup of the penicillins. It was discovered by scientists at Beecham and marketed as Pyopen. It has Gram-negative coverage which includes Pseudomonas aeruginosa but limited Gram-positive coverage. The carboxypenicillins are susceptible to degradation by beta-lactamase enzymes, although they are more resistant than ampicillin to degradation. Carbenicillin is also more stable at lower pH than ampicillin.
Production of antibiotics is a naturally occurring event, that thanks to advances in science can now be replicated and improved upon in laboratory settings. Due to the discovery of penicillin by Alexander Flemming, and the efforts of Florey and Chain in 1938, large-scale, pharmaceutical production of antibiotics has been made possible. As with the initial discovery of penicillin, most antibiotics have been discovered as a result of happenstance. Antibiotic production can be grouped into three methods: natural fermentation, semi-synthetic, and synthetic. As more and more bacteria continue to develop resistance to currently produced antibiotics, research and development of new antibiotics continues to be important. In addition to research and development into the production of new antibiotics, repackaging delivery systems is important to improving efficacy of the antibiotics that are currently produced. Improvements to this field have seen the ability to add antibiotics directly into implanted devices, aerosolization of antibiotics for direct delivery, and combination of antibiotics with non antibiotics to improve outcomes. The increase of antibiotic resistant strains of pathogenic bacteria has led to an increased urgency for the funding of research and development of antibiotics and a desire for production of new and better acting antibiotics.

Ticarcillin is a carboxypenicillin. It can be sold and used in combination with clavulanate as ticarcillin/clavulanic acid. Because it is a penicillin, it also falls within the larger class of beta-lactam antibiotics. Its main clinical use is as an injectable antibiotic for the treatment of Gram-negative bacteria, particularly Pseudomonas aeruginosa and Proteus vulgaris. It is also one of the few antibiotics capable of treating Stenotrophomonas maltophilia infections.
Ampicillin/sulbactam is a fixed-dose combination medication of the common penicillin-derived antibiotic ampicillin and sulbactam, an inhibitor of bacterial beta-lactamase. Two different forms of the drug exist. The first, developed in 1987 and marketed in the United States under the brand name Unasyn, generic only outside the United States, is an intravenous antibiotic. The second, an oral form called sultamicillin, is marketed under the brand name Ampictam outside the United States, and generic only in the United States. Ampicillin/sulbactam is used to treat infections caused by bacteria resistant to beta-lactam antibiotics. Sulbactam blocks the enzyme which breaks down ampicillin and thereby allows ampicillin to attack and kill the bacteria.
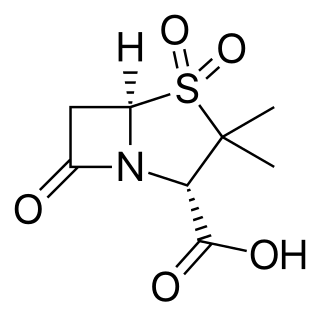
Sulbactam is a β-lactamase inhibitor. This drug is given in combination with β-lactam antibiotics to inhibit β-lactamase, an enzyme produced by bacteria that destroys the antibiotics.

Sultamicillin, sold under the brand name Unasyn among others, is an oral form of the penicillin antibiotic combination ampicillin/sulbactam. It is used for the treatment of bacterial infections of the upper and lower respiratory tract, the kidneys and urinary tract, skin and soft tissues, among other organs. It contains esterified ampicillin and sulbactam.

Flucloxacillin, also known as floxacillin, is an antibiotic used to treat skin infections, external ear infections, infections of leg ulcers, diabetic foot infections, and infection of bone. It may be used together with other medications to treat pneumonia, and endocarditis. It may also be used prior to surgery to prevent Staphylococcus infections. It is not effective against methicillin-resistant Staphylococcus aureus (MRSA). It is taken by mouth or given by injection into a vein or muscle.
Ampicillin/flucloxacillin (INNs) also known as co-fluampicil (BAN), and sold under the tradename Magnapen, is a combination drug of the two β-lactam antibiotics, ampicillin and flucloxacillin, both in equal amounts, available in a capsule and as a liquid, both taken by mouth, and as a formulation which can be given by injection into muscle or vein.

The Beecham Group plc was a British pharmaceutical company. It was once a constituent of the FTSE 100 Index. Beecham, after having merged with American pharmaceutical company SmithKline Beckman to become SmithKline Beecham, merged with Glaxo Wellcome to become GlaxoSmithKline (GSK). GSK still uses the Beechams brand name in the UK for its over-the-counter cold and flu relief products.

Bacampicillin (INN) is a penicillin antibiotic. It is a prodrug of ampicillin with improved oral bioavailability.

Cardiobacterium hominis is a Gram-negative bacillus (rod-shaped) bacterium commonly grouped with other bacteria into the HACEK group. It is one of several bacteria that is normally present in the mouth and upper part of the respiratory tract such as nose and throat. However, it may also rarely cause endocarditis, an infection of the heart valves.

Talampicillin is a beta lactam antibiotic from the penicillin family. It is an acid stable prodrug that was administered orally. It is not approved by the FDA for use in the United States. It should be avoided in Liver diseases

The aminopenicillins are a group of antibiotics in the penicillin family that are structural analogs of ampicillin. Like other penicillins and beta-lactam antibiotics, they contain a beta-lactam ring that is crucial to its antibacterial activity.
ATCvet code QJ51Antibacterials for intramammary use is a therapeutic subgroup of the Anatomical Therapeutic Chemical Classification System for veterinary medicinal products, a system of alphanumeric codes developed by the World Health Organization (WHO) for the classification of drugs and other medical products for veterinary use. Subgroup QJ51 is part of the anatomical group QJ Antiinfectives for systemic use.