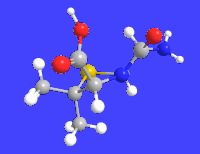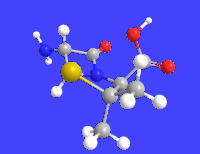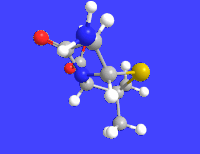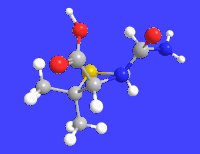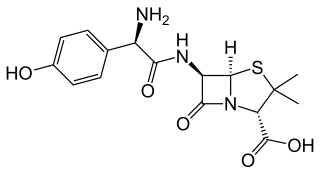
Amoxicillin is an antibiotic medication used to treat a number of bacterial infections. These include middle ear infection, strep throat, pneumonia, skin infections, and urinary tract infections among others. It is taken by mouth, or less commonly by injection.

A beta-lactam (β-lactam) ring is a four-membered lactam. A lactam is a cyclic amide, and beta-lactams are named so because the nitrogen atom is attached to the β-carbon atom relative to the carbonyl. The simplest β-lactam possible is 2-azetidinone. β-lactams are significant structural units of medicines as manifested in many β-lactam antibiotics Up to 1970, most β-lactam research was concerned with the penicillin and cephalosporin groups, but since then, a wide variety of structures have been described.

Beta-lactamases, (β-lactamases) are enzymes produced by bacteria that provide multi-resistance to beta-lactam antibiotics such as penicillins, cephalosporins, cephamycins, monobactams and carbapenems (ertapenem), although carbapenems are relatively resistant to beta-lactamase. Beta-lactamase provides antibiotic resistance by breaking the antibiotics' structure. These antibiotics all have a common element in their molecular structure: a four-atom ring known as a beta-lactam (β-lactam) ring. Through hydrolysis, the enzyme lactamase breaks the β-lactam ring open, deactivating the molecule's antibacterial properties.
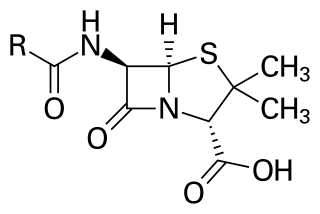
Penicillins are a group of β-lactam antibiotics originally obtained from Penicillium moulds, principally P. chrysogenum and P. rubens. Most penicillins in clinical use are synthesised by P. chrysogenum using deep tank fermentation and then purified. A number of natural penicillins have been discovered, but only two purified compounds are in clinical use: penicillin G and penicillin V. Penicillins were among the first medications to be effective against many bacterial infections caused by staphylococci and streptococci. They are still widely used today for different bacterial infections, though many types of bacteria have developed resistance following extensive use.
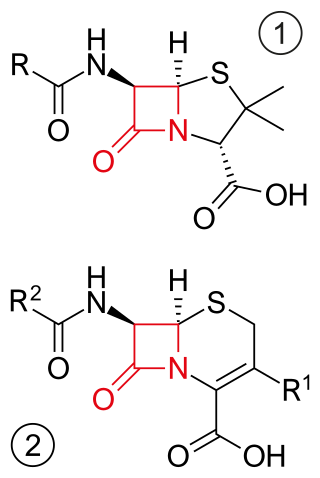
β-lactam antibiotics are antibiotics that contain a beta-lactam ring in their chemical structure. This includes penicillin derivatives (penams), cephalosporins and cephamycins (cephems), monobactams, carbapenems and carbacephems. Most β-lactam antibiotics work by inhibiting cell wall biosynthesis in the bacterial organism and are the most widely used group of antibiotics. Until 2003, when measured by sales, more than half of all commercially available antibiotics in use were β-lactam compounds. The first β-lactam antibiotic discovered, penicillin, was isolated from a strain of Penicillium rubens.

Methicillin (USAN), also known as meticillin (INN), is a narrow-spectrum β-lactam antibiotic of the penicillin class.

The cephalosporins are a class of β-lactam antibiotics originally derived from the fungus Acremonium, which was previously known as Cephalosporium.
DD-transpeptidase is a bacterial enzyme that catalyzes the transfer of the R-L-aca-D-alanyl moiety of R-L-aca-D-alanyl-D-alanine carbonyl donors to the γ-OH of their active-site serine and from this to a final acceptor. It is involved in bacterial cell wall biosynthesis, namely, the transpeptidation that crosslinks the peptide side chains of peptidoglycan strands.
A lactam is a cyclic amide, formally derived from an amino alkanoic acid. The term is a portmanteau of the words lactone + amide.

Ticarcillin is a carboxypenicillin. It can be sold and used in combination with clavulanate as ticarcillin/clavulanic acid. Because it is a penicillin, it also falls within the larger class of beta-lactam antibiotics. Its main clinical use is as an injectable antibiotic for the treatment of Gram-negative bacteria, particularly Pseudomonas aeruginosa and Proteus vulgaris. It is also one of the few antibiotics capable of treating Stenotrophomonas maltophilia infections.

Flucloxacillin, also known as floxacillin, is an antibiotic used to treat skin infections, external ear infections, infections of leg ulcers, diabetic foot infections, and infection of bone. It may be used together with other medications to treat pneumonia, and endocarditis. It may also be used prior to surgery to prevent Staphylococcus infections. It is not effective against methicillin-resistant Staphylococcus aureus (MRSA). It is taken by mouth or given by injection into a vein or muscle.

Dicloxacillin is a narrow-spectrum β-lactam antibiotic of the penicillin class. It is used to treat infections caused by susceptible (non-resistant) Gram-positive bacteria. It is active against beta-lactamase-producing organisms such as Staphylococcus aureus, which would otherwise be resistant to most penicillins. Dicloxacillin is available under a variety of trade names including Diclocil (BMS).
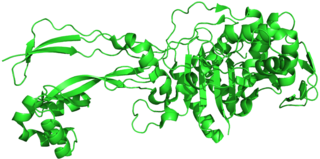
Penicillin-binding proteins (PBPs) are a group of proteins that are characterized by their affinity for and binding of penicillin. They are a normal constituent of many bacteria; the name just reflects the way by which the protein was discovered. All β-lactam antibiotics bind to PBPs, which are essential for bacterial cell wall synthesis. PBPs are members of a subgroup of enzymes called transpeptidases. Specifically, PBPs are DD-transpeptidases.

Oxacillin is a narrow-spectrum beta-lactam antibiotic of the penicillin class developed by Beecham.

The history of penicillin follows observations and discoveries of evidence of antibiotic activity of the mould Penicillium that led to the development of penicillins that became the first widely used antibiotics. Following the production of a relatively pure compound in 1942, penicillin was the first naturally-derived antibiotic.

The Beecham Group plc was a British pharmaceutical company. It was once a constituent of the FTSE 100 Index. Beecham, after having merged with American pharmaceutical company SmithKline Beckman to become SmithKline Beecham, merged with Glaxo Wellcome to become GlaxoSmithKline (GSK). GSK still uses the Beechams brand name in the UK for its over-the-counter cold and flu relief products.
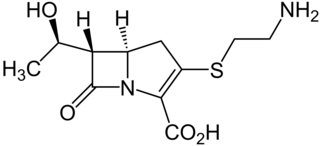
Thienamycin is one of the most potent naturally produced antibiotics known thus far, discovered in Streptomyces cattleya in 1976. Thienamycin has excellent activity against both Gram-positive and Gram-negative bacteria and is resistant to bacterial β-lactamase enzymes. Thienamycin is a zwitterion at pH 7.

John Clark Sheehan was an American organic chemist whose work on synthetic penicillin led to tailor-made forms of the drug. After nine years of hard work at the Massachusetts Institute of Technology (M.I.T.), he became the first to discover a practical method for synthesizing penicillin V. While achieving total synthesis, Sheehan also produced an intermediate compound, 6-aminopenicillanic acid, which turned out to be the foundation of hundreds of kinds of synthetic penicillin. Dr. Sheehan's research on synthetic penicillin paved the way for the development of customized forms of the lifesaving antibiotic that target specific bacteria. Over the four decades he worked at M.I.T., Sheehan came to hold over 30 patents, including the invention of ampicillin, a commonly used semi-synthetic penicillin that is taken orally rather than by injection. His research covered not only penicillin, but also peptides, other antibiotics, alkaloids, and steroids.
Cephalosporins are a broad class of bactericidal antibiotics that include the β-lactam ring and share a structural similarity and mechanism of action with other β-lactam antibiotics. The cephalosporins have the ability to kill bacteria by inhibiting essential steps in the bacterial cell wall synthesis which in the end results in osmotic lysis and death of the bacterial cell. Cephalosporins are widely used antibiotics because of their clinical efficiency and desirable safety profile.
Dr Frank Ralph Batchelor, known as Ralph, was a British biochemist and businessman.