
Levofloxacin, sold under the brand name Levaquin among others, is a broad-spectrum antibiotic of the fluoroquinolone drug class. It is the left-handed isomer of the medication ofloxacin. It is used to treat a number of bacterial infections including acute bacterial sinusitis, pneumonia, H. pylori, urinary tract infections, Legionnaires' disease, chronic bacterial prostatitis, and some types of gastroenteritis. Along with other antibiotics it may be used to treat tuberculosis, meningitis, or pelvic inflammatory disease. It is available by mouth, intravenously, and in eye drop form.

Clindamycin is a lincosamide antibiotic medication used for the treatment of a number of bacterial infections, including osteomyelitis (bone) or joint infections, pelvic inflammatory disease, strep throat, pneumonia, acute otitis media, and endocarditis. It can also be used to treat acne, and some cases of methicillin-resistant Staphylococcus aureus (MRSA). In combination with quinine, it can be used to treat malaria. It is available by mouth, by injection into a vein, and as a cream or a gel to be applied to the skin or in the vagina.

Aztreonam, sold under the brand name Azactam among others, is an antibiotic used primarily to treat infections caused by gram-negative bacteria such as Pseudomonas aeruginosa. This may include bone infections, endometritis, intra abdominal infections, pneumonia, urinary tract infections, and sepsis. It is given by intravenous or intramuscular injection or by inhalation.

Campylobacteriosis is among the most common infections caused by a bacterium in humans, often as a foodborne illness. It is caused by the Campylobacter bacterium, most commonly C. jejuni. It produces an inflammatory, sometimes bloody, diarrhea or dysentery syndrome, and usually cramps, fever and pain.

Colistin, also known as polymyxin E, is an antibiotic medication used as a last-resort treatment for multidrug-resistant Gram-negative infections including pneumonia. These may involve bacteria such as Pseudomonas aeruginosa, Klebsiella pneumoniae, or Acinetobacter. It comes in two forms: colistimethate sodium can be injected into a vein, injected into a muscle, or inhaled, and colistin sulfate is mainly applied to the skin or taken by mouth. Colistimethate sodium is a prodrug; it is produced by the reaction of colistin with formaldehyde and sodium bisulfite, which leads to the addition of a sulfomethyl group to the primary amines of colistin. Colistimethate sodium is less toxic than colistin when administered parenterally. In aqueous solutions, it undergoes hydrolysis to form a complex mixture of partially sulfomethylated derivatives, as well as colistin. Resistance to colistin began to appear as of 2015.

Amoxicillin/clavulanic acid, also known as co-amoxiclav or amox-clav, sold under the brand name Augmentin, among others, is an antibiotic medication used for the treatment of a number of bacterial infections. It is a combination consisting of amoxicillin, a β-lactam antibiotic, and potassium clavulanate, a β-lactamase inhibitor. It is specifically used for otitis media, streptococcal pharyngitis, pneumonia, cellulitis, urinary tract infections, and animal bites. It is taken by mouth or by injection into a vein.

Tigecycline, sold under the brand name Tygacil, is a tetracycline antibiotic medication for a number of bacterial infections. It is a glycylcycline class drug that is administered intravenously. It was developed in response to the growing rate of antibiotic resistant bacteria such as Staphylococcus aureus, Acinetobacter baumannii, and E. coli. As a tetracycline derivative antibiotic, its structural modifications has expanded its therapeutic activity to include Gram-positive and Gram-negative organisms, including those of multi-drug resistance.

Tobramycin is an aminoglycoside antibiotic derived from Streptomyces tenebrarius that is used to treat various types of bacterial infections, particularly Gram-negative infections. It is especially effective against species of Pseudomonas.

Cefepime is a fourth-generation cephalosporin antibiotic. Cefepime has an extended spectrum of activity against Gram-positive and Gram-negative bacteria, with greater activity against both types of organism than third-generation agents. A 2007 meta-analysis suggested when data of trials were combined, mortality was increased in people treated with cefepime compared with other β-lactam antibiotics. In response, the U.S. Food and Drug Administration (FDA) performed their own meta-analysis which found no mortality difference.

Marbofloxacin is a carboxylic acid derivative third generation fluoroquinolone antibiotic. It is used in veterinary medicine under the brand names Marbocyl, Forcyl, Marbo vet and Zeniquin. A formulation of marbofloxacin combined with clotrimazole and dexamethasone is available under the name Aurizon.

Cefoxitin is a second-generation cephamycin antibiotic developed by Merck & Co., Inc. from Cephamycin C in the year following its discovery, 1972. It was synthesized in order to create an antibiotic with a broader spectrum. It is often grouped with the second-generation cephalosporins. Cefoxitin requires a prescription and as of 2010 is sold under the brand name Mefoxin by Bioniche Pharma, LLC. The generic version of cefoxitin is known as cefoxitin sodium.

Cefodizime is a 3rd generation cephalosporin antibiotic with broad spectrum activity against aerobic gram positive and gram negative bacteria. Clinically, it has been shown to be effective against upper and lower respiratory tract infections, urinary tract infections, and gonorrhea. Cefodizime is a bactericidal antibiotic that targets penicillin-binding proteins (PBPs) 1A/B, 2, and 3 resulting in the eventual death of the bacterial cell. In vivo experimental models of infection showed that bacterial clearance by this drug is at least as effective compared with other 3rd generation cephalosporins. It has similar adverse effect profile to other 3rd generation cephalosporins as well, mainly being limited to gastrointestinal or dermatological side effects.

Prulifloxacin is an older synthetic antibiotic of the fluoroquinolone class undergoing clinical trials prior to a possible NDA submission to the U.S. Food and Drug Administration (FDA). It is a prodrug which is metabolized in the body to the active compound ulifloxacin. It was developed over two decades ago by Nippon Shinyaku Co. and was patented in Japan in 1987 and in the United States in 1989.
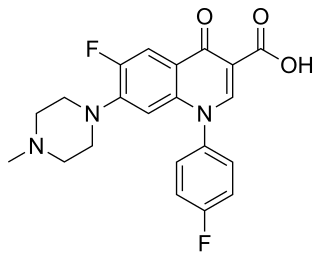
Difloxacin (INN), marketed under the trade name Dicural, is a second-generation, synthetic fluoroquinolone antibiotic used in veterinary medicine. It has broad-spectrum, concentration dependent, bactericidal activity; however, its efficacy is not as good as enrofloxacin or pradofloxacin.

Pradofloxacin, sold under the brand name Veraflox among others, is a third-generation enhanced spectrum veterinary antibiotic of the fluoroquinolone class. It was developed by Elanco Animal Health GmbH and received approval from the European Commission in April 2011, for prescription-only use in veterinary medicine for the treatment of bacterial infections in dogs and cats.
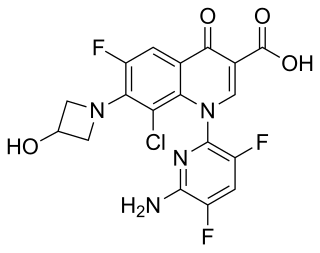
Delafloxacin sold under the brand name Baxdela among others, is a fluoroquinolone antibiotic used to treat acute bacterial skin and skin structure infections.
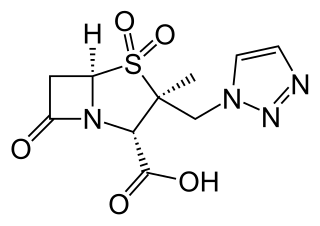
Ceftolozane/tazobactam, sold under the brand name Zerbaxa, (Merck) is a fixed-dose combination antibiotic medication used for the treatment of complicated urinary tract infections and complicated intra-abdominal infections in adults. Ceftolozane is a cephalosporin antibiotic, developed for the treatment of infections with gram-negative bacteria that are resistant to conventional antibiotics. It was studied for urinary tract infections, intra-abdominal infections and ventilator-associated bacterial pneumonia.

Finafloxacin (Xtoro) is a fluoroquinolone antibiotic. In the United States, it is approved by the Food and Drug Administration to treat acute otitis externa caused by the bacteria Pseudomonas aeruginosa and Staphylococcus aureus.
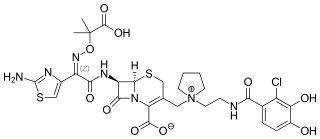
Cefiderocol, sold under the brand name Fetroja among others, is an antibiotic used to treat complicated urinary tract infections when no other options are available. It is indicated for the treatment of multi-drug-resistant Gram-negative bacteria including Pseudomonas aeruginosa. It is given by injection into a vein.



















