DNA topoisomerases are enzymes that catalyze changes in the topological state of DNA, interconverting relaxed and supercoiled forms, linked (catenated) and unlinked species, and knotted and unknotted DNA. Topological issues in DNA arise due to the intertwined nature of its double-helical structure, which, for example, can lead to overwinding of the DNA duplex during DNA replication and transcription. If left unchanged, this torsion would eventually stop the DNA or RNA polymerases involved in these processes from continuing along the DNA helix. A second topological challenge results from the linking or tangling of DNA during replication. Left unresolved, links between replicated DNA will impede cell division. The DNA topoisomerases prevent and correct these types of topological problems. They do this by binding to DNA and cutting the sugar-phosphate backbone of either one or both of the DNA strands. This transient break allows the DNA to be untangled or unwound, and, at the end of these processes, the DNA backbone is resealed. Since the overall chemical composition and connectivity of the DNA do not change, the DNA substrate and product are chemical isomers, differing only in their topology.
DNA gyrase, or simply gyrase, is an enzyme within the class of topoisomerase and is a subclass of Type II topoisomerases that reduces topological strain in an ATP dependent manner while double-stranded DNA is being unwound by elongating RNA-polymerase or by helicase in front of the progressing replication fork. It is the only known enzyme to actively contribute negative supercoiling to DNA, while it also is capable of relaxing positive supercoils. It does so by looping the template to form a crossing, then cutting one of the double helices and passing the other through it before releasing the break, changing the linking number by two in each enzymatic step. This process occurs in bacteria, whose single circular DNA is cut by DNA gyrase and the two ends are then twisted around each other to form supercoils. Gyrase is also found in eukaryotic plastids: it has been found in the apicoplast of the malarial parasite Plasmodium falciparum and in chloroplasts of several plants. Bacterial DNA gyrase is the target of many antibiotics, including nalidixic acid, novobiocin, albicidin, and ciprofloxacin.
Topoisomerase IV is one of two Type II topoisomerases in bacteria, the other being DNA gyrase. Like gyrase, topoisomerase IV is able to pass one double-strand of DNA through another double-strand of DNA, thereby changing the linking number of DNA by two in each enzymatic step. Both share a hetero-4-mer structure formed by a symmetric homodimer of A/B heterodimers, usually named ParC and ParE.
Production of antibiotics is a naturally occurring event, that thanks to advances in science can now be replicated and improved upon in laboratory settings. Due to the discovery of penicillin by Alexander Fleming, and the efforts of Florey and Chain in 1938, large-scale, pharmaceutical production of antibiotics has been made possible. As with the initial discovery of penicillin, most antibiotics have been discovered as a result of happenstance. Antibiotic production can be grouped into three methods: natural fermentation, semi-synthetic, and synthetic. As more and more bacteria continue to develop resistance to currently produced antibiotics, research and development of new antibiotics continues to be important. In addition to research and development into the production of new antibiotics, repackaging delivery systems is important to improving efficacy of the antibiotics that are currently produced. Improvements to this field have seen the ability to add antibiotics directly into implanted devices, aerosolization of antibiotics for direct delivery, and combination of antibiotics with non antibiotics to improve outcomes. The increase of antibiotic resistant strains of pathogenic bacteria has led to an increased urgency for the funding of research and development of antibiotics and a desire for production of new and better acting antibiotics.

Novobiocin, also known as albamycin, is an aminocoumarin antibiotic that is produced by the actinomycete Streptomyces niveus, which has recently been identified as a subjective synonym for S. spheroides a member of the class Actinomycetia. Other aminocoumarin antibiotics include clorobiocin and coumermycin A1. Novobiocin was first reported in the mid-1950s.

Norfloxacin, sold under the brand name Noroxin among others, is an antibiotic that belongs to the class of fluoroquinolone antibiotics. It is used to treat urinary tract infections, gynecological infections, inflammation of the prostate gland, gonorrhea and bladder infection. Eye drops were approved for use in children older than one year of age.
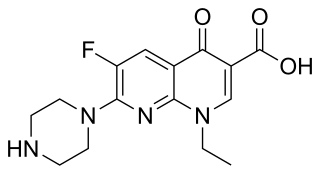
Enoxacin is an oral broad-spectrum fluoroquinolone antibacterial agent used in the treatment of urinary tract infections and gonorrhea. Insomnia is a common adverse effect. It is no longer available in the United States.

Sparfloxacin is a fluoroquinolone antibiotic used in the treatment of bacterial infections. It has a controversial safety profile.

Pefloxacin is a quinolone antibiotic used to treat bacterial infections. Pefloxacin has not been approved for use in the United States.

Cinoxacin is a quinolone antibiotic that has been discontinued in the U.K. as well the United States, both as a branded drug or a generic. The marketing authorization of cinoxacin has been suspended throughout the EU.
Topoisomerase inhibitors are chemical compounds that block the action of topoisomerases, which are broken into two broad subtypes: type I topoisomerases (TopI) and type II topoisomerases (TopII). Topoisomerase plays important roles in cellular reproduction and DNA organization, as they mediate the cleavage of single and double stranded DNA to relax supercoils, untangle catenanes, and condense chromosomes in eukaryotic cells. Topoisomerase inhibitors influence these essential cellular processes. Some topoisomerase inhibitors prevent topoisomerases from performing DNA strand breaks while others, deemed topoisomerase poisons, associate with topoisomerase-DNA complexes and prevent the re-ligation step of the topoisomerase mechanism. These topoisomerase-DNA-inhibitor complexes are cytotoxic agents, as the un-repaired single- and double stranded DNA breaks they cause can lead to apoptosis and cell death. Because of this ability to induce apoptosis, topoisomerase inhibitors have gained interest as therapeutics against infectious and cancerous cells.

Aminocoumarin is a class of antibiotics that act by an inhibition of the DNA gyrase enzyme involved in the cell division in bacteria. They are derived from Streptomyces species, whose best-known representative – Streptomyces coelicolor – was completely sequenced in 2002. The aminocoumarin antibiotics include:
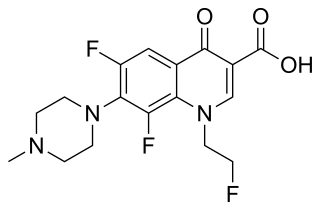
Fleroxacin is a quinolone antibiotic. It is sold under the brand names Quinodis and Megalocin.
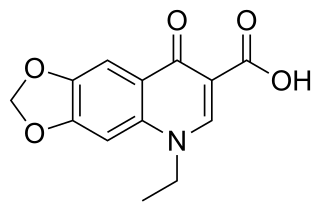
Oxolinic acid is a quinolone antibiotic developed in Japan in the 1970s. Dosages 12–20 mg/kg orally administered for five to ten days. The antibiotic works by inhibiting the enzyme DNA gyrase. It also acts as a dopamine reuptake inhibitor and has stimulant effects in mice.

Flumequine is a synthetic fluoroquinolone antibiotic used to treat bacterial infections. It is a first-generation fluoroquinolone antibacterial that has been removed from clinical use and is no longer being marketed. The marketing authorization of flumequine has been suspended throughout the EU. It kills bacteria by interfering with the enzymes that cause DNA to unwind and duplicate. Flumequine was used in veterinarian medicine for the treatment of enteric infections, as well as to treat cattle, swine, chickens, and fish, but only in a limited number of countries. It was occasionally used in France to treat urinary tract infections under the trade name Apurone. However this was a limited indication because only minimal serum levels were achieved.
A nucleic acid inhibitor is a type of antibacterial that acts by inhibiting the production of nucleic acids. There are two major classes: DNA inhibitors and RNA inhibitors. The antifungal flucytosine acts in a similar manner.

Amfonelic acid is a research chemical and dopaminergic stimulant with antibiotic properties. Although limited clinical trials have been conducted, it's primarily used in scientific research.

Quinolone antibiotics constitute a large group of broad-spectrum bacteriocidals that share a bicyclic core structure related to the substance 4-quinolone. They are used in human and veterinary medicine to treat bacterial infections, as well as in animal husbandry, specifically poultry production.

Neisseria gonorrhoeae, the bacterium that causes the sexually transmitted infection gonorrhea, has developed antibiotic resistance to many antibiotics. The bacteria was first identified in 1879.
The CcdA/CcdB Type II Toxin-antitoxin system is one example of the bacterial toxin-antitoxin (TA) systems that encode two proteins, one a potent inhibitor of cell proliferation (toxin) and the other its specific antidote (antitoxin). These systems preferentially guarantee growth of plasmid-carrying daughter cells in a bacterial population by killing newborn bacteria that have not inherited a plasmid copy at cell division.