 | |
| Clinical data | |
|---|---|
| Trade names | Priftin |
| Other names | 3{[(4-cyclopentyl-1-piperazinyl)imino]methyl}rifamycin |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a616011 |
| License data |
|
| Routes of administration | By mouth |
| Drug class | Macrolactam |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | increases when administered with food |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.057.021 |
| Chemical and physical data | |
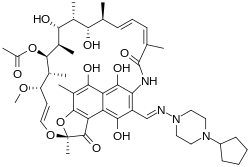
| Formula | C47H64N4O12 |
| Molar mass | 877.045 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 179 to 180 °C (354 to 356 °F) |
| |
| |
| (verify) | |
Rifapentine, sold under the brand name Priftin, is an antibiotic used in the treatment of tuberculosis. [2] In active tuberculosis it is used together with other antituberculosis medications. [2] In latent tuberculosis it is typically used with isoniazid. [2] It is taken by mouth. [2]
Contents
- Medical uses
- Adverse effects
- Pregnancy
- Contraindications
- Interactions
- Chemical structure
- History
- Society and culture
- Cancer-causing impurities
- References
- External links
Common side effects include low neutrophil counts in the blood, elevated liver enzymes, and white blood cells in the urine. [3] Serious side effects may include liver problems or Clostridioides difficile associated diarrhea. [3] It is unclear if use during pregnancy is safe. [3] Rifapentine is in the rifamycin family of medication and works by blocking DNA-dependent RNA polymerase. [3]
Rifapentine was approved for medical use in the United States in 1998. [2] It is on the World Health Organization's List of Essential Medicines. [4]