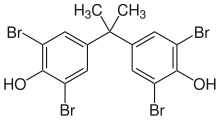
Polybrominated diphenyl ethers or PBDEs, are a class of organobromine compounds that are used as flame retardants. Like other brominated flame retardants, PBDEs have been used in a wide array of products, including building materials, electronics, furnishings, motor vehicles, airplanes, plastics, polyurethane foams, and textiles. They are structurally akin to polychlorinated diphenyl ethers (PCDEs), polychlorinated biphenyls (PCBs) and other polyhalogenated compounds, consisting of two halogenated aromatic rings. PBDEs are classified according to the average number of bromine atoms in the molecule. The health hazards of these chemicals have attracted increasing scrutiny, and they have been shown to reduce fertility in humans at levels found in households. Because of their toxicity and persistence, the industrial production of some PBDEs is restricted under the Stockholm Convention, a treaty to control and phase out major persistent organic pollutants (POPs).
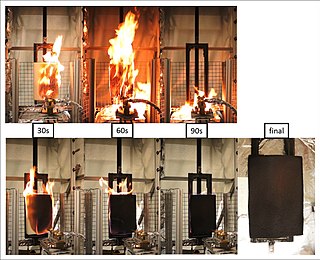
The term flame retardants subsumes a diverse group of chemicals that are added to manufactured materials, such as plastics and textiles, and surface finishes and coatings. Flame retardants are activated by the presence of an ignition source and are intended to prevent or slow the further development of ignition by a variety of different physical and chemical methods. They may be added as a copolymer during the polymerisation process, or later added to the polymer at a moulding or extrusion process or applied as a topical finish. Mineral flame retardants are typically additive while organohalogen and organophosphorus compounds can be either reactive or additive.
Endocrine disruptors, sometimes also referred to as hormonally active agents, endocrine disrupting chemicals, or endocrine disrupting compounds are chemicals that can interfere with endocrine systems. These disruptions can cause cancerous tumors, birth defects, and other developmental disorders. Found in many household and industrial products, endocrine disruptors "interfere with the synthesis, secretion, transport, binding, action, or elimination of natural hormones in the body that are responsible for development, behavior, fertility, and maintenance of homeostasis ."
Bisphenol A (BPA) is a chemical compound primarily used in the manufacturing of various plastics. It is a colourless solid which is soluble in most common organic solvents, but has very poor solubility in water. BPA is produced on an industrial scale by the condensation reaction of phenol and acetone. Global production in 2022 was estimated to be in the region of 10 million tonnes.

Persistent organic pollutants (POPs) are organic compounds that are resistant to degradation through chemical, biological, and photolytic processes. They are toxic chemicals that adversely affect human health and the environment around the world. Because they can be transported by wind and water, most POPs generated in one country can and do affect people and wildlife far from where they are used and released.
Brominated flame retardants (BFRs) are organobromine compounds that have an inhibitory effect on combustion chemistry and tend to reduce the flammability of products containing them. The brominated variety of commercialized chemical flame retardants comprise approximately 19.7% of the market. They are effective in plastics and textile applications like electronics, clothes and furniture.
Xenoestrogens are a type of xenohormone that imitates estrogen. They can be either synthetic or natural chemical compounds. Synthetic xenoestrogens include some widely used industrial compounds, such as PCBs, BPA, and phthalates, which have estrogenic effects on a living organism even though they differ chemically from the estrogenic substances produced internally by the endocrine system of any organism. Natural xenoestrogens include phytoestrogens which are plant-derived xenoestrogens. Because the primary route of exposure to these compounds is by consumption of phytoestrogenic plants, they are sometimes called "dietary estrogens". Mycoestrogens, estrogenic substances from fungi, are another type of xenoestrogen that are also considered mycotoxins.
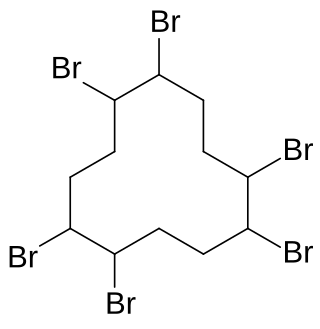
Hexabromocyclododecane is a brominated flame retardant. It consists of twelve carbon, eighteen hydrogen, and six bromine atoms tied to the ring. Its primary application is in extruded (XPS) and expanded (EPS) polystyrene foam that is used as thermal insulation in the building industry. Other uses are upholstered furniture, automobile interior textiles, car cushions and insulation blocks in trucks, packaging material, video cassette recorder housing and electric and electronic equipment. According to UNEP, "HBCD is produced in China, Europe, Japan, and the USA. The known current annual production is approximately 28,000 tonnes per year. The main share of the market volume is used in Europe and China". Due to its persistence, toxicity, and ecotoxicity, the Stockholm Convention on Persistent Organic Pollutants decided in May 2013 to list hexabromocyclododecane in Annex A to the convention with specific exemptions for production and use in expanded polystyrene and extruded polystyrene in buildings. Because HBCD has 16 possible stereo-isomers with different biological activities, the substance poses a difficult problem for manufacture and regulation.

Decabromodiphenyl ether is a brominated flame retardant which belongs to the group of polybrominated diphenyl ethers (PBDEs). It was commercialised in the 1970s and was initially thought to be safe, but is now recognised as a hazardous and persistent pollutant. It was added to Annex A of the Stockholm Convention on Persistent Organic Pollutants in 2017, which means that treaty members must take measures to eliminate its production and use. The plastics industry started switching to decabromodiphenyl ethane as an alternative in the 1990s, but this is now also coming under regulatory pressure due to concerns over human health.
Pentabromodiphenyl ether is a brominated flame retardant which belongs to the group of polybrominated diphenyl ethers (PBDEs). Because of their toxicity and persistence, their industrial production is to be eliminated under the Stockholm Convention, a treaty to control and phase out major persistent organic pollutants (POP).
Octabromodiphenyl ether is a brominated flame retardant which belongs to the group of polybrominated diphenyl ethers (PBDEs).

Triphenyl phosphate (TPhP) is the chemical compound with the formula OP(OC6H5)3. It is the simplest aromatic organophosphate. This colourless solid is the ester (triester) of phosphoric acid and phenol. It is used as a plasticizer and a fire retardant in a wide variety of settings and products.

Bisphenol S (BPS) is an organic compound with the formula (HOC6H4)2SO2. It has two phenol functional groups on either side of a sulfonyl group. It is commonly used in curing fast-drying epoxy resin adhesives. It is classified as a bisphenol, and a close molecular analog of bisphenol A (BPA). BPS differentiates from BPA by possessing a sulfone group (SO2) as the central linker of the molecule instead of a dimethylmethylene group (C 2), which is the case of bisphenol A.

Bisphenol A diglycidyl ether is an organic compound and is a liquid epoxy resin. The compound is a colorless viscous liquid. It is a key component of many epoxy resin formulations. Addition of further Bisphenol A and a catalyst and heat can produce Bisphenol A glycidyl ether epoxy resins of higher molecular weight that are solid.

Tris(1,3-dichloroisopropyl)phosphate (TDCPP) is a chlorinated organophosphate. Organophosphate chemicals have a wide variety of applications and are used as flame retardants, pesticides, plasticizers, and nerve gases. TDCPP is structurally similar to several other organophosphate flame retardants, such as tris(2-chloroethyl) phosphate (TCEP) and tris(chloropropyl)phosphate (TCPP). TDCPP and these other chlorinated organophosphate flame retardants are all sometimes referred to as "chlorinated tris".
Xenohormones or environmental hormones produced outside of the human body which exhibit endocrine hormone-like properties. They may be either of natural origin, such as phytoestrogens, which are derived from plants, or of synthetic origin. These compounds are able to activate the same endocrine receptors as their natural counterparts and are thus frequently implicated in endocrine disruption. The most commonly occurring xenohormones are xenoestrogens, which mimic the effects of estrogen. Other xenohormones include xenoandrogens and xenoprogesterones. Xenohormones are used for a variety of purposes including contraceptive & hormonal therapies, and agriculture. However, exposure to certain xenohormones early in childhood development can lead to a host of developmental issues including infertility, thyroid complications, and early onset of puberty. Exposure to others later in life has been linked to increased risks of testicular, prostate, ovarian, and uterine cancers.

Bisphenol F is an organic compound with the chemical formula (HOC
6H
4)
2CH
2. It is structurally related to bisphenol A (BPA), a popular precursor for forming plastics, as both belong to the category of molecules known as bisphenols, which feature two phenol groups connected via a linking group. In BPF, the two aromatic rings are linked by a methylene connecting group. In response to concern about the health effects of BPA, BPF is increasingly used as a substitute for BPA.

Bisphenol A controversy centers on concerns and debates about the biomedical significance of bisphenol A (BPA), which is a precursor to polymers that are used in some consumer products, including some food containers. The concerns began with the hypothesis that BPA is an endocrine disruptor, i.e. it mimics endocrine hormones and thus has the unintended and possibly far-reaching effects on people in physical contact with the chemical.

Bis(2-ethylhexyl)tetrabromophthalate (or TBPH), is a brominated phthalate derivative with the formula C24H34Br4O4 commonly used as a brominated flame retardant (BFR).

Gerald A. LeBlanc is an American biologist, toxicologist, author, and academic. He is a Professor Emeritus in the Department of Biological Sciences at the North Carolina State University.