Related Research Articles
Polybrominated diphenyl ethers or PBDEs, are a class of organobromine compounds that are used as flame retardants. Like other brominated flame retardants, PBDEs have been used in a wide array of products, including building materials, electronics, furnishings, motor vehicles, airplanes, plastics, polyurethane foams, and textiles. They are structurally akin to polychlorinated diphenyl ethers (PCDEs), polychlorinated biphenyls (PCBs) and other polyhalogenated compounds, consisting of two halogenated aromatic rings. PBDEs are classified according to the average number of bromine atoms in the molecule. The life-saving benefits of fire retardants led to their popularization. Standards for mass transit vehicles continues to increase as of 2021.
Flame retardants are a diverse group of chemicals that are added to manufactured materials, such as plastics and textiles, and surface finishes and coatings. Flame retardants are activated by the presence of an ignition source and prevent or slow the further development of flames by a variety of different physical and chemical mechanisms. They may be added as a copolymer during the polymerisation process, or later added to the polymer at a moulding or extrusion process or applied as a topical finish. Mineral flame retardants are typically additive, while organohalogen and organophosphorus compounds can be either reactive or additive.
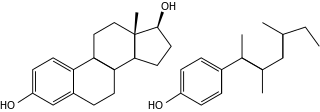
Endocrine disruptors, sometimes also referred to as hormonally active agents, endocrine disrupting chemicals, or endocrine disrupting compounds are chemicals that can interfere with endocrine systems. These disruptions can cause numerous adverse human health outcomes, including alterations in sperm quality and fertility; abnormalities in sex organs‚ endometriosis‚ early puberty‚ altered nervous system or immune function; certain cancers; respiratory problems; metabolic issues; diabetes, obesity, or cardiovascular problems; growth, neurological and learning disabilities, and more. Found in many household and industrial products, endocrine disruptors "interfere with the synthesis, secretion, transport, binding, action, or elimination of natural hormones in the body that are responsible for development, behavior, fertility, and maintenance of homeostasis ."
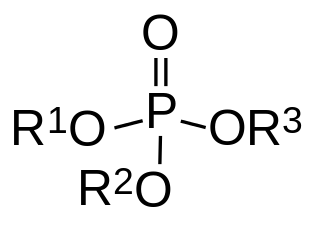
In organic chemistry, organophosphates are a class of organophosphorus compounds with the general structure O=P(OR)3, a central phosphate molecule with alkyl or aromatic substituents. They can be considered as esters of phosphoric acid. Organophosphates are best known for their use as pesticides.
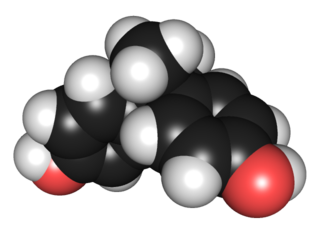
Bisphenol A (BPA) is a chemical compound primarily used in the manufacturing of various plastics. It is a colourless solid which is soluble in most common organic solvents, but has very poor solubility in water. BPA is produced on an industrial scale by the condensation reaction of phenol and acetone. Global production in 2022 was estimated to be in the region of 10 million tonnes.
A bromide ion is the negatively charged form (Br−) of the element bromine, a member of the halogens group on the periodic table. Most bromides are colorless. Bromides have many practical roles, being found in anticonvulsants, flame-retardant materials, and cell stains. Although uncommon, chronic toxicity from bromide can result in bromism, a syndrome with multiple neurological symptoms. Bromide toxicity can also cause a type of skin eruption, see potassium bromide. The bromide ion has an ionic radius of 196 pm.

Polybrominated biphenyls (PBBs), also called brominated biphenyls or polybromobiphenyls, are a group of manufactured chemicals that consist of polyhalogenated derivatives of a biphenyl core. Their chlorine analogs are the PCBs. While once widely used commercially, PBBs are now controlled substances under the Restriction of Hazardous Substances Directive, which limits their use in electrical and electronic products sold in the EU.
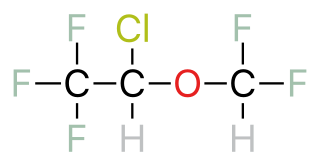
Halogenated ethers are a subcategory of ethers—organic chemicals that containan oxygen atom connected to two alkyl groups or similar structures. An example of an ether is the solvent diethyl ether. Halogenated ethers differ from other ethers because there are one or more halogen atoms—fluorine, chlorine, bromine, or iodine—as substituents on the carbon groups.. Examples of commonly used halogenated ethers include isoflurane, sevofluorane and desflurane.

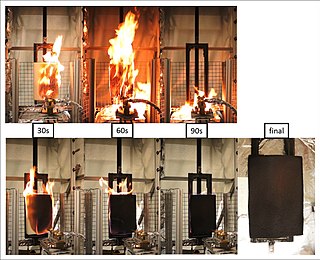
A fire retardant is a substance that is used to slow down or stop the spread of fire or reduce its intensity. This is commonly accomplished by chemical reactions that reduce the flammability of fuels or delay their combustion. Fire retardants may also cool the fuel through physical action or endothermic chemical reactions. Fire retardants are available as powder, to be mixed with water, as fire-fighting foams and fire-retardant gels. Fire retardants are also available as coatings or sprays to be applied to an object.
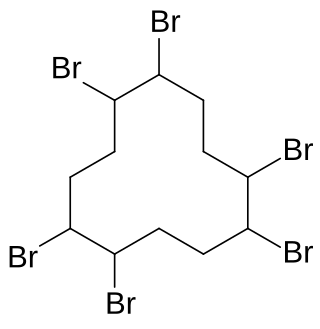
Hexabromocyclododecane is a brominated flame retardant. It consists of twelve carbon, eighteen hydrogen, and six bromine atoms tied to the ring. Its primary application is in extruded (XPS) and expanded (EPS) polystyrene foam used as thermal insulation in construction. Other uses are upholstered furniture, automobile interior textiles, car cushions and insulation blocks in trucks, packaging material, video cassette recorder housing, and electric and electronic equipment. According to UNEP, "HBCD is produced in China, Europe, Japan, and the USA. The last known current annual production is approximately 28,000 tonnes per year. The main share of the market volume is used in Europe and China". Due to its persistence, toxicity, and ecotoxicity, the Stockholm Convention on Persistent Organic Pollutants decided in May 2013 to list hexabromocyclododecane in Annex A to the convention with specific exemptions for production and use in expanded polystyrene and extruded polystyrene in buildings. Because HBCD has 16 possible stereo-isomers with different biological activities, the substance poses a difficult problem for manufacture and regulation.

Decabromodiphenyl ether is a brominated flame retardant which belongs to the group of polybrominated diphenyl ethers (PBDEs). It was commercialised in the 1970s and was initially thought to be safe, but is now recognised as a hazardous and persistent pollutant. It was added to Annex A of the Stockholm Convention on Persistent Organic Pollutants in 2017, which means that treaty members must take measures to eliminate its production and use. The plastics industry started switching to decabromodiphenyl ethane as an alternative in the 1990s, but this is now also coming under regulatory pressure due to concerns over human health.
Pentabromodiphenyl ether is a brominated flame retardant which belongs to the group of polybrominated diphenyl ethers (PBDEs). Because of their toxicity and persistence, their industrial production is to be eliminated under the Stockholm Convention, a treaty to control and phase out major persistent organic pollutants (POP).
Octabromodiphenyl ether is a brominated flame retardant which belongs to the group of polybrominated diphenyl ethers (PBDEs).
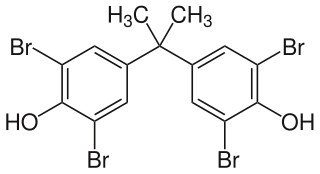
Tetrabromobisphenol A (TBBPA) is a brominated flame retardant. The compound is a white solid, although commercial samples appear yellow. It is one of the most common flame retardants.

Diphenyl ether is the organic compound with the formula (C6H5)2O. It is a colorless, low-melting solid. This, the simplest diaryl ether, has a variety of niche applications.
Organobromine chemistry is the study of the synthesis and properties of organobromine compounds, also called organobromides, which are organic compounds that contain carbon bonded to bromine. The most pervasive is the naturally produced bromomethane.

Susan D. Shaw was an American environmental health scientist, marine toxicologist, explorer, ocean conservationist, and author. A Doctor of Public Health, she was a professor in the Department of Environmental Health Sciences at the School of Public Health at the State University of New York at Albany, and Founder/President of the Shaw Institute, a nonprofit scientific institution with a mission to improve human and ecological health through innovative science and strategic partnerships. Shaw is globally recognized for pioneering high-impact environmental research on ocean pollution, climate change, oil spills, and plastics that has fueled public policy over three decades. In 1983, with landscape photographer Ansel Adams, she published Overexposure, the first book to document the health hazards of photographic chemicals. Shaw is credited as the first scientist to show that brominated flame retardant chemicals used in consumer products have contaminated marine mammals and commercially important fish stocks in the northwest Atlantic Ocean. She became the first scientist to dive into the Gulf of Mexico oil slick following the 2010 BP Deepwater Horizon oil rig explosion to investigate the impacts of chemical dispersants used in response to the spill.

Decabromodiphenyl ethane is a chemical compound used as a brominated flame retardant. It was commercialised in the 1990s as an alternative for decabromodiphenyl ether, following safety concern over that compound. The two molecules are chemically very similar, which gives them a similar application profile. Decabromodiphenyl ethane is now also coming under regulatory pressure.

Bis(2-ethylhexyl)tetrabromophthalate (or TBPH), is a brominated phthalate derivative with the formula C24H34Br4O4 commonly used as a brominated flame retardant (BFR).

Tetrabromobisphenol A diglycidyl ether is an epoxy resin consisting of tetrabromobisphenol A with ether linkages to two epichlorohydrin groups. An alernative structural comparison is as brominated form of bisphenol A diglycidyl ether. It is a brominated aromatic chemical used principally for giving flame retardant properties to materials. It is TSCA and REACH registered and has the molecular formula C21H20Br4O4. The IUPAC name is 2-{[2,6-dibromo-4-(2-{3,5-dibromo-4-[(oxiran-2-yl)methoxy]phenyl}propan-2-yl)phenoxy]methyl}oxirane.
References
- ↑ Townsend Solutions Estimate, "Flammschutz Online - the flame retardants market". Archived from the original on 2016-03-04. Retrieved 2014-10-26.
- ↑ Michael J. Dagani, Henry J. Barda, Theodore J. Benya, David C. Sanders: Bromine Compounds, Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi : 10.1002/14356007.a04_405
- ↑ The European Commission (9 February 2017). "Commission Regulation (EU) 2017/227". Official Journal of the European Union. L35: 6–9. Retrieved 16 June 2017.
- ↑ The final decision is available on the UNEP Stockholm Convention website here: "COP Decisions". Archived from the original on 2014-09-25. Retrieved 2014-10-26.
- ↑ "EU Risk Assessment Report of 2,2',6,6'-tetrabromo-4,4'-isopropylidenediphenol (tetrabromobisphenol-A or TBBP-A) Part II – human health" (PDF). Institute for Health and Consumer Protection. Archived from the original (PDF) on 2014-09-05. Retrieved 2014-10-26.
- ↑ Pedro Arias (2001): Brominated flame retardants – an overview. The Second International Workshop on Brominated Flame Retardants, Stockholm
- ↑ Beard, Adrian; Battenberg, Christian; Sutker, Burton J. (2021). "Flame Retardants". Ullmann's Encyclopedia of Industrial Chemistry. pp. 1–26. doi:10.1002/14356007.a11_123.pub2. ISBN 978-3-527-30385-4.
- 1 2 3 4 5 "Public Health Statement for PBDEs". Agency for Toxic Substances and Disease Registry, US Centers for Diesease Control and Prevention. 8 May 2017. Retrieved 24 December 2024.
- 1 2 "Polybrominated diphenyl ethers (PBDEs) - information sheet". Health Canada, Government of Canada. 14 September 2023. Retrieved 24 December 2024.
- 1 2 EFSA Panel on Contaminants in the Food Chain (24 January 2024). "Update of the risk assessment of polybrominated diphenyl ethers (PBDEs) in food". EFSA Journal. 22 (1): e8497. doi:10.2903/j.efsa.2024.8497.