
Diphosgene is a chemical compound with the formula ClCO2CCl3. This colorless liquid is a valuable reagent in the synthesis of organic compounds. Diphosgene is related to phosgene and has comparable toxicity, but is more conveniently handled because it is a liquid, whereas phosgene is a gas.
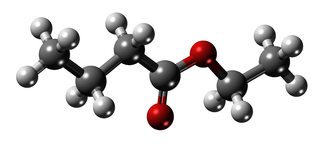
Ethyl butyrate, also known as ethyl butanoate, or butyric ether, is an ester with the chemical formula CH3CH2CH2COOCH2CH3. It is soluble in propylene glycol, paraffin oil, and kerosene. It has a fruity odor, similar to pineapple and is a key ingredient used as a flavor enhancer in processed orange juices. It also occurs naturally in many fruits, albeit at lower concentrations.

Isobutyric acid, also known as 2-methylpropanoic acid, is a carboxylic acid with structural formula (CH3)2CHCOOH. It is a colorless liquid with a somewhat unpleasant odor. It is soluble in water and organic solvents. Isobutyric acid is an isomer of n-butyric acid. Deprotonation or esterification of isobutyric acid gives derivatives called isobutyrates. Isobutyric acid is found in the free state in carobs (Ceratonia siliqua), in vanilla, and in the root of Arnica dulcis, and as an ethyl ester in croton oil.

Ethyl acetate is the organic compound with the formula CH
3–COO–CH
2–CH
3, simplified to C
4H
8O
2. This colorless liquid has a characteristic sweet smell and is used in glues, nail polish removers, decaffeinating tea and coffee. Ethyl acetate is the ester of ethanol and acetic acid; it is manufactured on a large scale for use as a solvent. The combined annual production in 1985 of Japan, North America, and Europe was about 400,000 tonnes. In 2004, an estimated 1.3 million tonnes were produced worldwide.

Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula (CH3CO)2O. Commonly abbreviated Ac2O, it is the simplest isolable anhydride of a carboxylic acid and is widely used as a reagent in organic synthesis. It is a colorless liquid that smells strongly of acetic acid, which is formed by its reaction with moisture in the air.
Anisole, or methoxybenzene, is an organic compound with the formula CH3OC6H5. It is a colorless liquid with a smell reminiscent of anise seed, and in fact many of its derivatives are found in natural and artificial fragrances. The compound is mainly made synthetically and is a precursor to other synthetic compounds. It is an ether.
Adipoyl chloride (or adipoyl dichloride) is the organic compound with the formula (CH2CH2C(O)Cl)2. It is a colorless liquid. It reacts with water to give adipic acid.

An organic acid anhydride is an acid anhydride that is an organic compound. An acid anhydride is a compound that has two acyl groups bonded to the same oxygen atom. A common type of organic acid anhydride is a carboxylic anhydride, where the parent acid is a carboxylic acid, the formula of the anhydride being (RC(O))2O. Symmetrical acid anhydrides of this type are named by replacing the word acid in the name of the parent carboxylic acid by the word anhydride. Thus, (CH3CO)2O is called acetic anhydride. Mixed (or unsymmetrical) acid anhydrides, such as acetic formic anhydride (see below), are known.

Maleic anhydride is an organic compound with the formula C2H2(CO)2O. It is the acid anhydride of maleic acid. It is a colorless or white solid with an acrid odor. It is produced industrially on a large scale for applications in coatings and polymers.

Butyraldehyde, also known as butanal, is an organic compound with the formula CH3(CH2)2CHO. This compound is the aldehyde derivative of butane. It is a colourless flammable liquid with an acrid smell. It is miscible with most organic solvents.

Crotonic acid ((2E)-but-2-enoic acid) or is a short-chain unsaturated carboxylic acid, described by the formula CH3CH=CHCO2H. It is called crotonic acid because it was erroneously thought to be a saponification product of croton oil. It crystallizes as colorless needles from hot water. The cis-isomer of crotonic acid is called isocrotonic acid. Crotonic acid is soluble in water and many organic solvents. Its odor is similar to butyric acid.

Triflic acid, also known as trifluoromethanesulfonic acid, TFMS, TFSA, HOTf or TfOH, is a sulfonic acid with the chemical formula CF3SO3H. It is one of the strongest acids. Triflic acid is mainly used in research as a catalyst for esterification. It is a hygroscopic, colorless, slightly viscous liquid and is soluble in polar solvents.

Succinic anhydride, is an organic compound with the molecular formula (CH2CO)2O. This colorless solid is the acid anhydride of succinic acid.

The bioconversion of biomass to mixed alcohol fuels can be accomplished using the MixAlco process. Through bioconversion of biomass to a mixed alcohol fuel, more energy from the biomass will end up as liquid fuels than in converting biomass to ethanol by yeast fermentation.

Propanoic anhydride is an organic compound with the formula (CH3CH2CO)2O. This simple acid anhydride is a colourless liquid. It is a widely used reagent in organic synthesis.
Acetyl iodide is an organoiodine compound with the formula CH3COI. It is a colourless liquid. It is formally derived from acetic acid. Although far rarer in the laboratory than the related acetyl bromide and acetyl chloride, acetyl iodide is produced, transiently at least, on a far larger scale than any other acid halide. Specifically, it is generated by the carbonylation of methyl iodide in the Cativa and Monsanto processes that are the main industrial route to acetic acid. It is also an intermediate in the production of acetic anhydride from methyl acetate.

Acetic acid, systematically named ethanoic acid, is a colourless liquid organic compound with the chemical formula CH3COOH (also written as CH3CO2H or C2H4O2). When undiluted, it is sometimes called glacial acetic acid. Vinegar is no less than 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water. Acetic acid has a distinctive sour taste and pungent smell. In addition to household vinegar, it is mainly produced as a precursor to polyvinyl acetate and cellulose acetate. It is classified as a weak acid since it only partially dissociates in solution, but concentrated acetic acid is corrosive and can attack the skin.
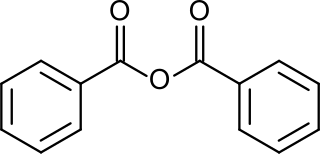
Benzoic anhydride is the organic compound with the formula (C6H5CO)2O. It is acid anhydride of benzoic acid and the simplest symmetrical aromatic acid anhydride. It is a white solid.
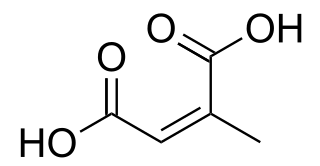
Citraconic acid is an organic compound with the formula CH3C2H(CO2H)2. It is a white solid. It is the cis-isomer of mesaconic acid. It is one of the pyrocitric acids formed upon the heating of citric acid. Citraconic acid can be produced, albeit inefficiently, by oxidation of xylene and methylbutanols. The acid displays the unusual property of spontaneously forming the anhydride, which, unlike maleic anhydride, is a liquid at room temperature.

Acetyl nitrate is the organic compound with the formula CH3C(O)ONO2. It is classified as the mixed anhydride of nitric and acetic acids. It is a colorless explosive liquid that fumes in moist air.



















