Contents
- Preparation and handling
- Storage and purification
- Biogenic iodomethane
- Reactions
- Methylation reagent
- Other reactions
- Trideuteroiodomethane
- Use as a pesticide
- United States
- Safety
- Toxicity and biological effects
- Carcinogenicity in mammals
- See also
- References
- Additional sources
- External links
 | |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name Iodomethane [1] | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol) | |||
| Abbreviations |
| ||
| 969135 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.745 | ||
| EC Number |
| ||
| 1233 | |||
| KEGG | |||
| MeSH | methyl+iodide | ||
PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
| UN number | 2644 | ||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
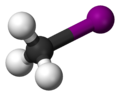
| CH3I | |||
| Molar mass | 141.939 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Odor | Pungent, ether-like [2] | ||
| Density | 2.28 g/mL | ||
| Melting point | −66.5 °C; −87.6 °F; 206.7 K | ||
| Boiling point | 42.4 to 42.8 °C; 108.2 to 108.9 °F; 315.5 to 315.9 K | ||
| 14 g/L (at 20 °C, 68 °F) [3] | |||
| log P | 1.609 | ||
| Vapor pressure | 54.4 kPa (at 20 °C, 68 °F) | ||
Henry's law constant (kH) | 1.4 μmol/(Pa·kg) | ||
| −57.2×10−6 cm3/mol | |||
Refractive index (nD) | 1.530–1.531 | ||
| Structure | |||
| Tetrahedron | |||
| Thermochemistry | |||
Heat capacity (C) | 82.75 J/(K·mol) | ||
Std enthalpy of formation (ΔfH⦵298) | −14.1 to −13.1 kJ/mol | ||
Std enthalpy of combustion (ΔcH⦵298) | −808.9 to −808.3 kJ/mol | ||
| Hazards | |||
| GHS labelling: | |||
  | |||
| Danger | |||
| H301, H312, H315, H331, H335, H351 | |||
| P261, P280, P301+P310, P311 | |||
| NFPA 704 (fire diamond) | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
| ||
LC50 (median concentration) |
| ||
LCLo (lowest published) | 3800 ppm (rat, 15 min) [4] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) | TWA 5 ppm (28 mg/m3) [skin] [2] | ||
REL (Recommended) | Ca TWA 2 ppm (10 mg/m3) [skin] [2] | ||
IDLH (Immediate danger) | Ca [100 ppm] [2] | ||
| Related compounds | |||
Related iodomethanes | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Iodomethane, also called methyl iodide, and commonly abbreviated "MeI", is the chemical compound with the formula CH3I. It is a dense, colorless, volatile liquid. In terms of chemical structure, it is related to methane by replacement of one hydrogen atom by an atom of iodine. It is naturally emitted in small amounts by rice plantations. [5] It is also produced in vast quantities estimated to be greater than 214,000 tons annually by algae and kelp in the world's temperate oceans, and in lesser amounts on land by terrestrial fungi and bacteria. It is used in organic synthesis as a source of methyl groups.