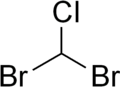 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name Dibromo(chloro)methane | |
Other names
| |
| Identifiers | |
3D model (JSmol) | |
| Abbreviations | CDBM[ citation needed ] |
| 1731046 | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.277 |
| EC Number |
|
| KEGG | |
| MeSH | chlorodibromomethane |
PubChem CID | |
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| CHBr2Cl | |
| Molar mass | 208.28 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 2.451 g mL−1 |
| Melting point | −22 °C (−8 °F; 251 K) |
| Boiling point | 119 to 120 °C (246 to 248 °F; 392 to 393 K) at 99.7 kPa |
| log P | 2.206 |
Henry's law constant (kH) | 8.6 μmol Pa−1 kg−1 |
| −75.1·10−6 cm3/mol | |
Refractive index (nD) | 1.547 |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H302 | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) | 370 mg kg−1(oral, rat) |
| Related compounds | |
Related alkanes | |
Related compounds | 2-Chloroethanol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Dibromochloromethane is a colorless to yellow, heavy and nonflammable compound with formula CHBr
2Cl. [1] [2] It is a trihalomethane. The substance has a sweet odour. [3] Small quantities of dibromochloromethane are produced in ocean by algae.[ citation needed ]