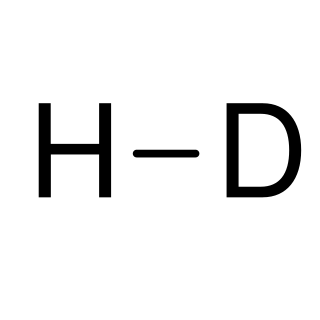Deuterium (hydrogen-2, symbol 2H or D, also known as heavy hydrogen) is one of two stable isotopes of hydrogen (the other is protium, or hydrogen-1). The deuterium nucleus, called a deuteron, contains one proton and one neutron, whereas the far more common protium has no neutrons in the nucleus. Deuterium has a natural abundance in Earth's oceans of about one atom of deuterium among every 6,420 atoms of hydrogen. Thus deuterium accounts for about 0.0156% by number (0.0312% by mass) of all hydrogen in the oceans: 4.85×1013 tonnes of deuterium – mainly in form of HOD (or 1HO2H or 1H2HO) and only rarely in form of D2O (or 2H2O) – in 1.4×1018 tonnes of water. The abundance of deuterium changes slightly from one kind of natural water to another (see Vienna Standard Mean Ocean Water).

Heavy water is a form of water whose hydrogen atoms are all deuterium rather than the common hydrogen-1 isotope that makes up most of the hydrogen in normal water. The presence of the heavier isotope gives the water different nuclear properties, and the increase in mass gives it slightly different physical and chemical properties when compared to normal water.

Dichloromethane is an organochlorine compound with the formula CH2Cl2. This colorless, volatile liquid with a chloroform-like, sweet odor is widely used as a solvent. Although it is not miscible with water, it is slightly polar, and miscible with many organic solvents.

Semiheavy water is the result of replacing one of the protium in light water with deuterium. It exists whenever there is water with light hydrogen (protium, 1H) and deuterium (D or 2H) in the mix. This is because hydrogen atoms (hydrogen-1 and deuterium) are rapidly exchanged between water molecules. Water containing 50% H and 50% D in its hydrogen contains about 50% HDO and 25% each of H2O and D2O, in dynamic equilibrium. In regular water, about 1 molecule in 3,200 is HDO (one hydrogen in 6,400 is D). By comparison, heavy water D2O occurs at a proportion of about 1 molecule in 41 million (i.e., one in 6,4002). This makes semiheavy water far more common than "normal" heavy water.
In physical organic chemistry, a kinetic isotope effect (KIE) is the change in the reaction rate of a chemical reaction when one of the atoms in the reactants is replaced by one of its isotopes. Formally, it is the ratio of rate constants for the reactions involving the light (kL) and the heavy (kH) isotopically substituted reactants (isotopologues):

Hydrogen (1H) has three naturally occurring isotopes, sometimes denoted 1
H
, 2
H
, and 3
H
. 1
H
and 2
H
are stable, while 3
H
has a half-life of 12.32(2) years. Heavier isotopes also exist, all of which are synthetic and have a half-life of less than one zeptosecond (10−21 s). Of these, 5
H
is the least stable, while 7
H
is the most.
Pyrylium is a cation with formula C5H5O+, consisting of a six-membered ring of five carbon atoms, each with one hydrogen atom, and one positively charged oxygen atom. The bonds in the ring are conjugated as in benzene, giving it an aromatic character. In particular, because of the positive charge, the oxygen atom is trivalent. Pyrilium is a mono-cyclic and heterocyclic compound, one of the oxonium ions.
Hydrogen–deuterium exchange is a chemical reaction in which a covalently bonded hydrogen atom is replaced by a deuterium atom, or vice versa. It can be applied most easily to exchangeable protons and deuterons, where such a transformation occurs in the presence of a suitable deuterium source, without any catalyst. The use of acid, base or metal catalysts, coupled with conditions of increased temperature and pressure, can facilitate the exchange of non-exchangeable hydrogen atoms, so long as the substrate is robust to the conditions and reagents employed. This often results in perdeuteration: hydrogen-deuterium exchange of all non-exchangeable hydrogen atoms in a molecule.
In chemistry, the hydron, informally called proton, is the cationic form of atomic hydrogen, represented with the symbol H+
. The general term "hydron", endorsed by IUPAC, encompasses cations of hydrogen regardless of isotope: thus it refers collectively to protons (1H+) for the protium isotope, deuterons (2H+ or D+) for the deuterium isotope, and tritons (3H+ or T+) for the tritium isotope.
Deuterated chloroform, also known as chloroform-d, is the organic compound with the formula CDCl3 or C2HCl3. Deuterated chloroform is a common solvent used in NMR spectroscopy. The properties of C2HCl3 and ordinary CHCl3 (chloroform) are virtually identical.

Deuterated acetone ((CD3)2CO), also known as acetone-d6, is a form (isotopologue) of acetone (CH3)2CO in which the hydrogen atom (H) is replaced with deuterium (heavy hydrogen) isotope (2H or D). Deuterated acetone is a common solvent used in NMR spectroscopy.

Deuterated benzene (C6D6) is an isotopologue of benzene (C6H6) in which the hydrogen atom ("H") is replaced with deuterium (heavy hydrogen) isotope ("D").

Heavy isotope diet is the consumption of nutrients in which some atoms are replaced with their heavier non-radioactive isotopes, such as deuterium(2H) or heavy carbon (13C). Biomolecules that incorporate heavier isotopes give rise to more stable molecular structures under certain circumstances, which is hypothesized to increase resistance to damage associated with ageing or diseases.
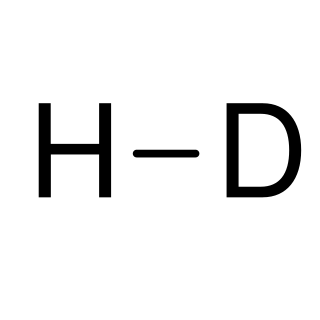
Hydrogen deuteride is an isotopologue of dihydrogen composed of two isotopes of hydrogen: the majority isotope 1H (protium) and 2H (deuterium). Its proper molecular formula is 1H2H, but for simplification, it is usually written as HD.

Deuterated solvents are a group of compounds where one or more hydrogen atoms are substituted by deuterium atoms.
Deuterium NMR is NMR spectroscopy of deuterium, an isotope of hydrogen. Deuterium is an isotope with spin = 1, unlike hydrogen-1, which has spin = 1/2. The term deuteron NMR, in direct analogy to proton NMR, is also used. Deuterium NMR has a range of chemical shift similar to proton NMR but with poor resolution, due to the smaller magnitude of the magnetic dipole moment of the deuteron relative to the proton. It may be used to verify the effectiveness of deuteration: a deuterated compound will show a strong peak in deuterium NMR but not proton NMR.

A deuterated drug is a small molecule medicinal product in which one or more of the hydrogen atoms in the drug molecule have been replaced by its heavier stable isotope deuterium. Because of the kinetic isotope effect, deuterium-containing drugs may have significantly lower rates of metabolism, and hence a longer half-life.

Kinetic isotope effect is observed when molecules containing heavier isotopes of the same elements engage in a chemical reaction at a slower rate. Deuterium-reinforced lipids can be used for protecting living cells by slowing the chain reaction of lipid peroxidation. The lipid bilayer of the cell and organelle membranes contain polyunsaturated fatty acids (PUFA) are key components of cell and organelle membranes. Any process that either increases oxidation of PUFAs or hinders their ability to be replaced can lead to serious disease. Correspondingly, drugs that stop the chain reaction of lipid peroxidation have preventive and therapeutic potential.

Sodium deuteroxide or deuterated sodium hydroxide is a chemical compound with the formula NaOD or NaO2H. It is a white solid very similar to sodium hydroxide, of which it is an isotopologue. It is used as a strong base and deuterium source in the production of other deuterated compounds. For example, reaction with chloral hydrate gives deuterated chloroform, and reaction with N-nitrosodimethylamine gives the deuterated analog of that compound. Sodium deuteroxide is an ionic compound, consisting of sodium cations Na+ and deuteroxide anions −OD (or −O2H).
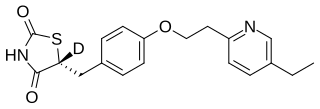
PXL065 (d-R-pioglitazone) is a drug candidate for the treatment of nonalcoholic steatohepatitis (NASH). It is the deuterium-stabilized (R)-enantiomer of pioglitazone which lacks PPARγ agonist activity and the associated side effects of weight gain and edema. PXL065 (formerly known as DRX-065) has demonstrated preclinical efficacy for both NASH and X-linked adrenoleukodystrophy (X-ALD). In 2022, it successfully completed a 9 month Phase 2 trial in biopsy-proven NASH patients where it met the primary endpoint for reduction in liver fat without weight gain or edema.