 | |
| Names | |
|---|---|
| Preferred IUPAC name Fluoro(iodo)methane | |
| Other names Fluoroiodomethane Fluoro-iodo-methane Fluoromethyl iodide | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.201.539 |
PubChem CID | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| CH2FI | |
| Molar mass | 159.93 g/mol |
| Boiling point | 53.4 °C (128.1 °F; 326.5 K) |
| Hazards | |
| GHS labelling: | |
 | |
| Danger | |
| H301, H311, H330 | |
| P260, P264, P270, P271, P280, P284, P301+P310, P302+P352, P304+P340, P310, P312, P320, P321, P322, P330, P361, P363, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
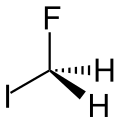
Fluoroiodomethane is the halomethane with the formula FCH2I. Also classified as a fluoroiodocarbon (FIC), it is a colorless liquid. It is a reagent for the introduction of the fluoromethyl (FCH2) group.