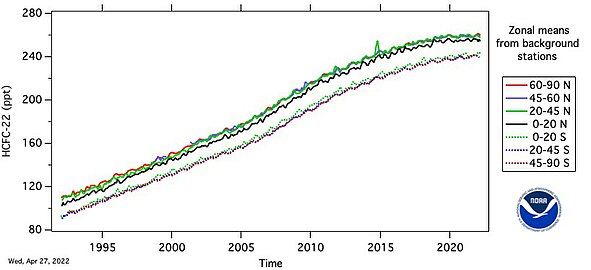
- HCFC-22 measured by the Advanced Global Atmospheric Gases Experiment (AGAGE) in the lower atmosphere (troposphere) at stations around the world. Abundances are given as pollution free monthly mean mole fractions in parts-per-trillion.
- Growth of R-22 (CFC-22) abundance in Earth's atmosphere since year 1992. [5]
Contents
- Production and current applications
- Environmental effects
- Phaseout in the European Union
- Phaseout in the United States
- R-22, retrofit using substitute refrigerants
- Physical properties
- Price history and availability
- References
- External links
| |||
 Liquefied chlorodifluoromethane boiling when exposed to ambient temperature and pressure. | |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name Chloro(difluoro)methane | |||
| Other names Chlorodifluoromethane Difluoromonochloromethane Monochlorodifluoromethane HCFC-22 R-22 Genetron 22 Freon 22 Arcton 4 Arcton 22 UN 1018 Difluorochloromethane Fluorocarbon-22 Refrigerant 22 | |||
| Identifiers | |||
3D model (JSmol) | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.793 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
| UN number | 1018 | ||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
| CHClF2 | |||
| Molar mass | 86.47 g/mol | ||
| Appearance | Colorless gas | ||
| Odor | Sweetish [1] | ||
| Density | 3.66 kg/m3 at 15 °C, gas | ||
| Melting point | −175.42 °C (−283.76 °F; 97.73 K) | ||
| Boiling point | −40.7 °C (−41.3 °F; 232.5 K) | ||
| 0.7799 vol/vol at 25 °C; 3.628 g/L | |||
| log P | 1.08 | ||
| Vapor pressure | 908 kPa at 20 °C | ||
Henry's law constant (kH) | 0.033 mol⋅kg−1⋅bar−1 | ||
| −38.6·10−6 cm3/mol | |||
| Structure | |||
| Tetrahedral | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards | Dangerous for the environment (N), Central nervous system depressant, Carc. Cat. 3 | ||
| GHS labelling: | |||
 | |||
| Warning | |||
| H420 | |||
| P202, P262, P271, P403 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | nonflammable [1] | ||
| 632 °C (1,170 °F; 905 K) | |||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) | None [1] | ||
REL (Recommended) | TWA 1000 ppm (3500 mg/m3) ST 1250 ppm (4375 mg/m3) [1] | ||
IDLH (Immediate danger) | N.D. [1] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
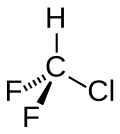
Chlorodifluoromethane or difluoromonochloromethane is a hydrochlorofluorocarbon (HCFC). This colorless gas is better known as HCFC-22, or R-22, or CHClF
2. It was commonly used as a propellant and refrigerant. These applications were phased out under the Montreal Protocol in developed countries in 2020 due to the compound's ozone depletion potential (ODP) and high global warming potential (GWP), and in developing countries this process will be completed by 2030. R-22 is a versatile intermediate in industrial organofluorine chemistry, e.g. as a precursor to tetrafluoroethylene.



![Shipping container for the gas in Japan. Container [( 2%3FT%3F )] GRPU 918195(3)---No,2 [( Pictures taken in Japan )] .jpg](http://upload.wikimedia.org/wikipedia/commons/thumb/8/8b/Container_%E3%80%90_2%3FT%3F_%E3%80%91_GRPU_918195%283%29---No%2C2_%E3%80%90_Pictures_taken_in_Japan_%E3%80%91.jpg/250px-Container_%E3%80%90_2%3FT%3F_%E3%80%91_GRPU_918195%283%29---No%2C2_%E3%80%90_Pictures_taken_in_Japan_%E3%80%91.jpg)

