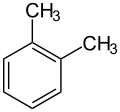
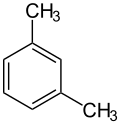
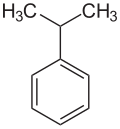
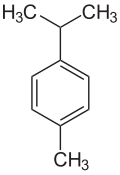
An alkylbenzene is a chemical compound that contains a monocyclic aromatic ring attaching to one or more saturated hydrocarbon chains. [1] Alkylbenzenes are derivatives of benzene, in which one or more hydrogen atoms are replaced by alkyl groups. The simplest member, toluene (or methylbenzene), has the hydrogen atom of the benzene ring replaced by a methyl group. The chemical formula of alkylbenzenes is CnH2n-6. [2]
Contents
- Examples
- Reactions
- Spectroscopy
- Production
- Safety hazards
- Application
- Synthetic sulfonates
- Solvent use
- Literature
- References
- References 2

Alkylbenzenes are a very important class of hydrocarbons, especially in the synthetic production industry. It is the raw material in the production of synthetic sulfonate detergents, which are found in a variety of household products such as soap, shampoo, toothpaste, laundry detergent, etc. Linear alkylbenzenes (LAB) and branched alkylbenzenes (BAB) are families of alkylbenzene used to prepare synthetic sulfonates. However, LABs are more industrially favoured since the discovery of its extensive biodegradable yield over BAB-based sulfonates in the 1960s. [3]