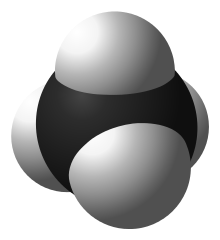
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic; their odor is usually faint, and may be similar to that of gasoline or lighter fluid. They occur in a diverse range of molecular structures and phases: they can be gases, liquids, low melting solids or polymers.

The halogens are a group in the periodic table consisting of six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and the radioactive elements astatine (At) and tennessine (Ts), though some authors would exclude tennessine as its chemistry is unknown and is theoretically expected to be more like that of gallium. In the modern IUPAC nomenclature, this group is known as group 17.

Chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) are fully or partly halogenated hydrocarbons that contain carbon (C), hydrogen (H), chlorine (Cl), and fluorine (F), produced as volatile derivatives of methane, ethane, and propane.
The haloalkanes are alkanes containing one or more halogen substituents. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes that contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula "RX" where R is an alkyl or substituted alkyl group and X is a halogen.

Fluorocarbons are chemical compounds with carbon-fluorine bonds. Compounds that contain many C-F bonds often have distinctive properties, e.g., enhanced stability, volatility, and hydrophobicity. Several fluorocarbons and their derivatives are commercial polymers, refrigerants, drugs, and anesthetics.

Carbon tetrachloride, also known by many other names (such as carbon tet for short and tetrachloromethane, also recognised by the IUPAC), is a chemical compound with the chemical formula CCl4. It is a non-flammable, dense, colourless liquid with a "sweet" chloroform-like odour that can be detected at low levels. It was formerly widely used in fire extinguishers, as a precursor to refrigerants, an anthelmintic and a cleaning agent, but has since been phased out because of environmental and safety concerns. Exposure to high concentrations of carbon tetrachloride can affect the central nervous system and degenerate the liver and kidneys. Prolonged exposure can be fatal.
Bromotrifluoromethane, commonly referred to by the code numbers Halon 1301, R13B1, Halon 13B1 or BTM, is an organic halide with the chemical formula CBrF3. It is used for gaseous fire suppression as a far less toxic alternative to bromochloromethane.
Chloromethane, also called methyl chloride, Refrigerant-40, R-40 or HCC 40, is an organic compound with the chemical formula CH3Cl. One of the haloalkanes, it is a colorless, sweet-smelling, flammable gas. Methyl chloride is a crucial reagent in industrial chemistry, although it is rarely present in consumer products, and was formerly utilized as a refrigerant. Most chloromethane is biogenic.
In chemistry, halogenation is a chemical reaction which introduces one or more halogens into a chemical compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polymers, drugs. This kind of conversion is in fact so common that a comprehensive overview is challenging. This article mainly deals with halogenation using elemental halogens. Halides are also commonly introduced using salts of the halides and halogen acids. Many specialized reagents exist for and introducing halogens into diverse substrates, e.g. thionyl chloride.
In organic chemistry, free-radical halogenation is a type of halogenation. This chemical reaction is typical of alkanes and alkyl-substituted aromatics under application of UV light. The reaction is used for the industrial synthesis of chloroform (CHCl3), dichloromethane (CH2Cl2), and hexachlorobutadiene. It proceeds by a free-radical chain mechanism.

Fluoromethane, also known as methyl fluoride, Freon 41, Halocarbon-41 and HFC-41, is a non-toxic, liquefiable, and flammable gas at standard temperature and pressure. It is made of carbon, hydrogen, and fluorine. The name stems from the fact that it is methane (CH4) with a fluorine atom substituted for one of the hydrogen atoms. It is used in semiconductor manufacturing processes as an etching gas in plasma etch reactors.
In chemistry, trihalomethanes (THMs) are chemical compounds in which three of the four hydrogen atoms of methane are replaced by halogen atoms. Trihalomethanes with all the same halogen atoms are called haloforms. Many trihalomethanes find uses in industry as solvents or refrigerants. Some THMs are also environmental pollutants, and a few are considered carcinogenic.

Oxygen fluorides are compounds of elements oxygen and fluorine with the general formula OnF2, where n = 1 to 6. Many different oxygen fluorides are known:
Fluoroform, or trifluoromethane, is the chemical compound with the formula CHF3. It is a hydrofluorocarbon as well as being a part of the haloforms, a class of compounds with the formula CHX3 with C3v symmetry. Fluoroform is used in diverse applications in organic synthesis. It is not an ozone depleter but is a greenhouse gas.

1,1,1,2,3,3,3-Heptafluoropropane, also called heptafluoropropane, HFC-227ea, HFC-227 or FM-200, as well as apaflurane (INN), is a colourless, odourless gaseous halocarbon commonly used as a gaseous fire suppression agent.
Trifluoroiodomethane, also referred to as trifluoromethyl iodide is a halomethane with the formula CF3I. It is an experimental alternative to Halon 1301 (CBrF3) in unoccupied areas. It would be used as a gaseous fire suppression flooding agent for in-flight aircraft and electronic equipment fires.
In chemistry, a reaction intermediate, or intermediate, is a molecular entity arising within the sequence of a stepwise chemical reaction. It is formed as the reaction product of an elementary step, from the reactants and/or preceding intermediates, but is consumed in a later step. It does not appear in the chemical equation for the overall reaction.

Bromochloromethane or methylene bromochloride and Halon 1011 is a mixed halomethane. It is a heavy low-viscosity liquid with refractive index 1.4808.

Dichlorofluoromethane or Freon 21 or R 21 is a halomethane or hydrochlorofluorocarbon with the formula CHCl2F. It is a colorless and odorless gas. It is produced by fluorination of chloroform using a catalyst such as antimony trifluoride:
Tetrahalomethanes are fully halogenated methane derivatives of general formula CFkCllBrmInAtp, where:Tetrahalomethanes are on the border of inorganic and organic chemistry, thus they can be assigned both inorganic and organic names by IUPAC: tetrafluoromethane - carbon tetrafluoride, tetraiodomethane - carbon tetraiodide, dichlorodifluoromethane - carbon dichloride difluoride.