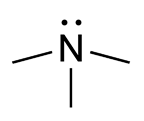
Trimethylaminuria (TMAU), also known as fish odor syndrome or fish malodor syndrome, is a rare metabolic disorder that causes a defect in the normal production of an enzyme named flavin-containing monooxygenase 3 (FMO3). When FMO3 is not working correctly or if not enough enzyme is produced, the body loses the ability to properly convert trimethylamine (TMA) from precursor compounds in food digestion into trimethylamine oxide (TMAO), through a process called N-oxidation. Trimethylamine then builds up and is released in the person's sweat, urine, and breath, giving off a strong fishy odor or strong body odor. Primary trimethylaminuria is caused by genetic mutations that affect the FMO3 function of the liver. A variant of TMAU is an acquired form of TMAU where there is no genetic cause, yet excessive TMA is secreted. TMAU2 can be caused by a range of issues, including a precursor overload, hormonal issues related to menstrual cycles, liver damage, and liver and kidney failure. It is hypothesised that intestinal dysbiosis may cause temporary TMAU2 symptoms, however there is no direct evidence of this in a scientific study.

Flavin-containing monooxygenase 3 (FMO3), also known as dimethylaniline monooxygenase [N-oxide-forming] 3 and trimethylamine monooxygenase, is a flavoprotein enzyme (EC 1.14.13.148) that in humans is encoded by the FMO3 gene. This enzyme catalyzes the following chemical reaction, among others:
Liver carboxylesterase 1 also known as carboxylesterase 1 is an enzyme that in humans is encoded by the CES1 gene. The protein is also historically known as serine esterase 1 (SES1), monocyte esterase and cholesterol ester hydrolase (CEH). Three transcript variants encoding three different isoforms have been found for this gene. The various protein products from isoform a, b and c range in size from 568, 567 and 566 amino acids long, respectively.

UDP-glucuronosyltransferase 1-10 is an enzyme that in humans is encoded by the UGT1A10 gene.

UDP-glucuronosyltransferase 2B15 is an enzyme that in humans is encoded by the UGT2B15 gene.

UDP-glucuronosyltransferase 1-4 is an enzyme that in humans is encoded by the UGT1A4 gene.

Equilibrative nucleoside transporter 2 (ENT2) is a protein that in humans is encoded by the SLC29A2 gene.

Dimethylaniline monooxygenase [N-oxide-forming] 5 is an enzyme that in humans is encoded by the FMO5 gene.

Dimethylaniline monooxygenase [N-oxide-forming] 2 is an enzyme that in humans is encoded by the FMO2 gene.

Cytochrome P450 2A13 is a protein that in humans is encoded by the CYP2A13 gene.

Cytochrome P450 3A43 is a protein that in humans is encoded by the CYP3A43 gene.

Bifunctional 3'-phosphoadenosine 5'-phosphosulfate synthetase 2 is an enzyme that in humans is encoded by the PAPSS2 gene.

Cytochrome P450 2F1 is a protein that in humans is encoded by the CYP2F1 gene.

Carbonyl reductase [NADPH] 3 is an enzyme that in humans is encoded by the CBR3 gene.

Dimethylaniline monooxygenase [N-oxide-forming] 4 is an enzyme that in humans is encoded by the FMO4 gene.

Solute carrier organic anion transporter family member 2B1 also known as organic anion-transporting polypeptide 2B1 (OATP2B1) is a protein that in humans is encoded by the gene SLCO2B1.

CYP2W1 is a protein that in humans is encoded by the CYP2W1 gene.

CYP2A7 is a protein that in humans is encoded by the CYP2A7 gene.

UDP-glucuronosyltransferase 1-9 is an enzyme that in humans is encoded by the UGT1A9 gene.

The flavin-containing monooxygenase (FMO) protein family specializes in the oxidation of xeno-substrates in order to facilitate the excretion of these compounds from living organisms. These enzymes can oxidize a wide array of heteroatoms, particularly soft nucleophiles, such as amines, sulfides, and phosphites. This reaction requires an oxygen, an NADPH cofactor, and an FAD prosthetic group. FMOs share several structural features, such as a NADPH binding domain, FAD binding domain, and a conserved arginine residue present in the active site. Recently, FMO enzymes have received a great deal of attention from the pharmaceutical industry both as a drug target for various diseases and as a means to metabolize pro-drug compounds into active pharmaceuticals. These monooxygenases are often misclassified because they share activity profiles similar to those of cytochrome P450 (CYP450), which is the major contributor to oxidative xenobiotic metabolism. However, a key difference between the two enzymes lies in how they proceed to oxidize their respective substrates; CYP enzymes make use of an oxygenated heme prosthetic group, while the FMO family utilizes FAD to oxidize its substrates.

















