 | |
| Names | |
|---|---|
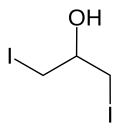
| Preferred IUPAC name 1,3-Diiodopropan-2-ol [1] | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.007.807 |
| EC Number |
|
| MeSH | 1,3-diiodo-2-propanol |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C3H6I2O | |
| Molar mass | 311.889 g·mol−1 |
| Pharmacology | |
| D08AG04 ( WHO ) | |
| Related compounds | |
Related compounds | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Diiodohydroxypropane is an antiseptic and disinfectant. It is also known as jothion [2] or iothion. [3]