Related Research Articles

The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs), and francium (Fr). Together with hydrogen they constitute group 1, which lies in the s-block of the periodic table. All alkali metals have their outermost electron in an s-orbital: this shared electron configuration results in their having very similar characteristic properties. Indeed, the alkali metals provide the best example of group trends in properties in the periodic table, with elements exhibiting well-characterised homologous behaviour. This family of elements is also known as the lithium family after its leading element.

Caesium is a chemical element; it has symbol Cs and atomic number 55. It is a soft, silvery-golden alkali metal with a melting point of 28.5 °C (83.3 °F), which makes it one of only five elemental metals that are liquid at or near room temperature. Caesium has physical and chemical properties similar to those of rubidium and potassium. It is pyrophoric and reacts with water even at −116 °C (−177 °F). It is the least electronegative element, with a value of 0.79 on the Pauling scale. It has only one stable isotope, caesium-133. Caesium is mined mostly from pollucite. Caesium-137, a fission product, is extracted from waste produced by nuclear reactors. It has the largest atomic radius of all elements whose radii have been measured or calculated, at about 260 picometers.
Carbon compounds are defined as chemical substances containing carbon. More compounds of carbon exist than any other chemical element except for hydrogen. Organic carbon compounds are far more numerous than inorganic carbon compounds. In general bonds of carbon with other elements are covalent bonds. Carbon is tetravalent but carbon free radicals and carbenes occur as short-lived intermediates. Ions of carbon are carbocations and carbanions are also short-lived. An important carbon property is catenation as the ability to form long carbon chains and rings.

Caesium fluoride or cesium fluoride is an inorganic compound with the formula CsF and it is a hygroscopic white salt. Caesium fluoride can be used in organic synthesis as a source of the fluoride anion. Caesium also has the highest electropositivity of all known elements and fluorine has the highest electronegativity of all known elements.

Caesium chloride or cesium chloride is the inorganic compound with the formula CsCl. This colorless salt is an important source of caesium ions in a variety of niche applications. Its crystal structure forms a major structural type where each caesium ion is coordinated by 8 chloride ions. Caesium chloride dissolves in water. CsCl changes to NaCl structure on heating. Caesium chloride occurs naturally as impurities in carnallite, sylvite and kainite. Less than 20 tonnes of CsCl is produced annually worldwide, mostly from a caesium-bearing mineral pollucite.
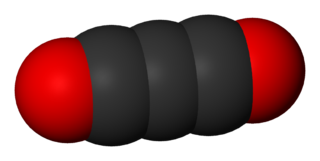
Carbon suboxide, or tricarbon dioxide, is an organic, oxygen-containing chemical compound with formula C3O2 and structure O=C=C=C=O. Its four cumulative double bonds make it a cumulene. It is one of the stable members of the series of linear oxocarbons O=Cn=O, which also includes carbon dioxide and pentacarbon dioxide. Although if carefully purified it can exist at room temperature in the dark without decomposing, it will polymerize under certain conditions.

Ozonide is the polyatomic anion O−3. Cyclic organic compounds formed by the addition of ozone to an alkene are also called ozonides.

Rubidium oxide is the chemical compound with the formula Rb2O. Rubidium oxide is highly reactive towards water, and therefore it would not be expected to occur naturally. The rubidium content in minerals is often calculated and quoted in terms of Rb2O. In reality, the rubidium is typically present as a component of (actually, an impurity in) silicate or aluminosilicate. A major source of rubidium is lepidolite, KLi2Al(Al,Si)3O10(F,OH)2, wherein Rb sometimes replaces K.

Germane is the chemical compound with the formula GeH4, and the germanium analogue of methane. It is the simplest germanium hydride and one of the most useful compounds of germanium. Like the related compounds silane and methane, germane is tetrahedral. It burns in air to produce GeO2 and water. Germane is a group 14 hydride.

Caesium chromate or cesium chromate is an inorganic compound with the formula Cs2CrO4. It is a yellow crystalline solid that is the caesium salt of chromic acid, and it crystallises in the orthorhombic system.
Suboxides are a class of oxides wherein the electropositive element is in excess relative to the “normal” oxides. When the electropositive element is a metal, the compounds are sometimes referred to as “metal-rich”. Thus the normal oxide of caesium is Cs2O, which is described as a Cs+ salt of O2−. A suboxide of caesium is Cs11O3, where the charge on Cs is clearly less than 1+, but the oxide is still described as O2−. Suboxides typically feature extensive bonding between the electropositive element, often leading to clusters.
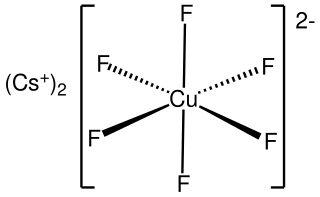
Caesium hexafluorocuprate is the inorganic compound with the chemical formula Cs
2CuF
6. It is a red solid that degrades upon contact with water. It was first prepared be heating CsCuCl
3 and caesium fluoride at 410°C under 350 atmospheres of fluorine:
Chromium pentafluoride is the inorganic compound with the chemical formula CrF5. It is a red volatile solid that melts at 34 °C. It is the highest known chromium fluoride, since the hypothetical chromium hexafluoride has not yet been synthesized.
Gallium(I) oxide, digallium monoxide or gallium suboxide is an inorganic compound with the formula Ga2O.

Caesium oxalate (standard IUPAC spelling) dicesium oxalate, or cesium oxalate (American spelling) is the oxalate of caesium. Caesium oxalate has the chemical formula of Cs2C2O4.

Caesium azide or cesium azide is an inorganic compound of caesium and nitrogen. It is a salt of azide with the formula CsN3.

Caesium peroxide or cesium peroxide is an inorganic compound of caesium and oxygen with the chemical formula Cs2O2. It can be formed from caesium metal by adding a stoichiometric amount in ammonia solution, or oxidizing the solid metal directly.
Caesium sesquioxide is a chemical compound with the formula Cs2O3 or Cs4O6. In terms of oxidation states, Caesium in this compound has a nominal charge of +1, and the oxygen is a mixed peroxide and superoxide for a structural formula of (Cs+)4(O−2)2(O2−2). Compared to the other caesium oxides, this phase is less well studied, but has been long present in the literature. It can be created by thermal decomposition of caesium superoxide at 290 °C.

Caesium superoxide is the superoxide of caesium. It is an orange solid.
References
- ↑ Simon, A. (1997), "Group 1 and 2 Suboxides and Subnitrides — Metals with Atomic Size Holes and Tunnels", Coord. Chem. Rev., 163: 253–270, doi:10.1016/S0010-8545(97)00013-1 .
- ↑ Sananda Chatterjee (2012). Encyclopedia Of Inorganic Chemistry Vol 2. Discovery publishing House Pvt. Ltd. p. 723. ISBN 978-81-8356-874-6.