
In chemistry, an ester is a functional group derived from an acid in which the hydrogen atom (H) of at least one acidic hydroxyl group of that acid is replaced by an organyl group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well, but not according to the IUPAC.

In organic chemistry, a ketone is an organic compound with the structure R−C(=O)−R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group −C(=O)−. The simplest ketone is acetone, with the formula (CH3)2CO. Many ketones are of great importance in biology and industry. Examples include many sugars (ketoses), many steroids, and the solvent acetone.

In organic chemistry, a ketene is an organic compound of the form RR'C=C=O, where R and R' are two arbitrary monovalent chemical groups. The name may also refer to the specific compound ethenone H2C=C=O, the simplest ketene.

Acetophenone is the organic compound with the formula C6H5C(O)CH3. It is the simplest aromatic ketone. This colorless, viscous liquid is a precursor to useful resins and fragrances.

In organic chemistry, a dicarbonyl is a molecule containing two carbonyl groups. Although this term could refer to any organic compound containing two carbonyl groups, it is used more specifically to describe molecules in which both carbonyls are in close enough proximity that their reactivity is changed, such as 1,2-, 1,3-, and 1,4-dicarbonyls. Their properties often differ from those of monocarbonyls, and so they are usually considered functional groups of their own. These compounds can have symmetrical or unsymmetrical substituents on each carbonyl, and may also be functionally symmetrical or unsymmetrical.

In organic chemistry, enols are a type of Functional group or intermediate in organic chemistry containing a group with the formula C=C(OH). The term enol is an abbreviation of alkenol, a portmanteau deriving from "-ene"/"alkene" and the "-ol". Many kinds of enols are known.

Acetylacetone is an organic compound with the chemical formula CH3−C(=O)−CH2−C(=O)−CH3. It is classified as a 1,3-diketone. It exists in equilibrium with a tautomer CH3−C(=O)−CH=C(−OH)−CH3. The mixture is a colorless liquid. These tautomers interconvert so rapidly under most conditions that they are treated as a single compound in most applications. Acetylacetone is a building block for the synthesis of many coordination complexes as well as heterocyclic compounds.
In chemistry, a dehydration reaction is a chemical reaction that involves the loss of water from the reacting molecule or ion. Dehydration reactions are common processes, the reverse of a hydration reaction.
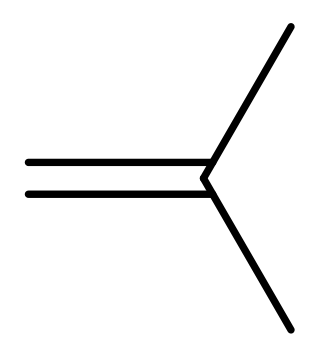
Isobutylene is a hydrocarbon with the chemical formula (CH3)2C=CH2. It is a four-carbon branched alkene (olefin), one of the four isomers of butylene. It is a colorless flammable gas, and is of considerable industrial value.

Cyclopentanone is the organic compound with the formula (CH2)4CO. This cyclic ketone is a colorless volatile liquid.

In organic chemistry an enol ether is an alkene with an alkoxy substituent. The general structure is R2C=CR-OR where R = H, alkyl or aryl. A common subfamily of enol ethers are vinyl ethers, with the formula ROCH=CH2. Important enol ethers include the reagent 3,4-dihydropyran and the monomers methyl vinyl ether and ethyl vinyl ether.

Methyl vinyl ketone (MVK, IUPAC name: butenone) is the organic compound with the formula CH3C(O)CH=CH2. It is a reactive compound classified as an enone, in fact the simplest example thereof. It is a colorless, flammable, highly toxic liquid with a pungent odor. It is soluble in water and polar organic solvents. It is a useful intermediate in the synthesis of other compounds.
In chemistry, carbonylation refers to reactions that introduce carbon monoxide (CO) into organic and inorganic substrates. Carbon monoxide is abundantly available and conveniently reactive, so it is widely used as a reactant in industrial chemistry. The term carbonylation also refers to oxidation of protein side chains.

Ethenone is the formal name for ketene, an organic compound with formula C2H2O or H2C=C=O. It is the simplest member of the ketene class. It is an important reagent for acetylations.
Methyl vinyl ether is an organic compound with the chemical formula CH3OCH=CH2. A colorless gas, it is the simplest enol ether. It is used as a synthetic building block, as is the related compound ethyl vinyl ether (a liquid at room temperature).
In industrial chemistry, carboalkoxylation is a process for converting alkenes to esters. This reaction is a form of carbonylation. A closely related reaction is hydrocarboxylation, which employs water in place of alcohols.

β-Isophorone is an organic compound with the formula (CH3)3C6H7O. Classified as a β,γ-unsaturated ketone, it is an isomer of and common impurity in the major industrial intermediate α-isophorone, which is produced from acetone. Like the alpha isomer, beta-isophorone is a colorless liquid.

Ethyl vinyl ether is an organic compound with the chemical formula CH3CH2OCH=CH2. It is the simplest enol ether that is liquid at room temperature. It is used as a synthetic building block and a monomer.

α,β-Unsaturated carbonyl compounds are organic compounds with the general structure (O=CR)−Cα=Cβ−R. Such compounds include enones and enals, but also carboxylic acids and the corresponding esters and amides. In these compounds, the carbonyl group is conjugated with an alkene. Unlike the case for carbonyls without a flanking alkene group, α,β-unsaturated carbonyl compounds are susceptible to attack by nucleophiles at the β-carbon. This pattern of reactivity is called vinylogous. Examples of unsaturated carbonyls are acrolein (propenal), mesityl oxide, acrylic acid, and maleic acid. Unsaturated carbonyls can be prepared in the laboratory in an aldol reaction and in the Perkin reaction.
Geranylacetone is an organic compound with the formula CH3C(O)(CH2)2CH=C(CH3)(CH2)2CH=C(CH3)2. A colorless oil, it is the product of coupling geranyl and acetonyl groups. It is a precursor to synthetic squalene.
















