Related Research Articles

Corundum is a crystalline form of aluminium oxide typically containing traces of iron, titanium, vanadium, and chromium. It is a rock-forming mineral. It is a naturally transparent material, but can have different colors depending on the presence of transition metal impurities in its crystalline structure. Corundum has two primary gem varieties: ruby and sapphire. Rubies are red due to the presence of chromium, and sapphires exhibit a range of colors depending on what transition metal is present. A rare type of sapphire, padparadscha sapphire, is pink-orange.

Inorganic chemistry deals with synthesis and behavior of inorganic and organometallic compounds. This field covers chemical compounds that are not carbon-based, which are the subjects of organic chemistry. The distinction between the two disciplines is far from absolute, as there is much overlap in the subdiscipline of organometallic chemistry. It has applications in every aspect of the chemical industry, including catalysis, materials science, pigments, surfactants, coatings, medications, fuels, and agriculture.

An oxide is a chemical compound containing at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– ion with oxygen in the oxidation state of −2. Most of the Earth's crust consists of oxides. Even materials considered pure elements often develop an oxide coating. For example, aluminium foil develops a thin skin of Al2O3 that protects the foil from further oxidation.
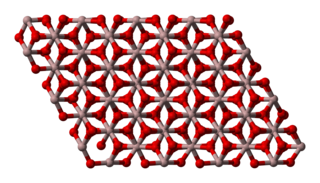
Aluminium oxide (or aluminium(III) oxide) is a chemical compound of aluminium and oxygen with the chemical formula Al2O3. It is the most commonly occurring of several aluminium oxides, and specifically identified as aluminium oxide. It is commonly called alumina and may also be called aloxide, aloxite, or alundum in various forms and applications. It occurs naturally in its crystalline polymorphic phase α-Al2O3 as the mineral corundum, varieties of which form the precious gemstones ruby and sapphire. Al2O3 is significant in its use to produce aluminium metal, as an abrasive owing to its hardness, and as a refractory material owing to its high melting point.
An acidic oxide is an oxide that either produces an acidic solution upon addition to water, or acts as an acceptor of hydroxide ions effectively functioning as a Lewis acid. Acidic oxides will typically have a low pKa and may be inorganic or organic. A commonly encountered acidic oxide, carbon dioxide produces an acidic solution when dissolved.
A trioxide is a compound with three oxygen atoms. For metals with the M2O3 formula there are several common structures. Al2O3, Cr2O3, Fe2O3, and V2O3 adopt the corundum structure. Many rare earth oxides adopt the "A-type rare earth structure" which is hexagonal. Several others plus indium oxide adopt the "C-type rare earth structure", also called "bixbyite", which is cubic and related to the fluorite structure.

Chromium trioxide (also known as chromium(VI) oxide or chromic anhydride) is an inorganic compound with the formula CrO3. It is the acidic anhydride of chromic acid, and is sometimes marketed under the same name. This compound is a dark-purple solid under anhydrous conditions and bright orange when wet. The substance dissolves in water concomitant with hydrolysis. Millions of kilograms are produced annually, mainly for electroplating. Chromium trioxide is a powerful oxidiser, a mutagen, and a carcinogen.
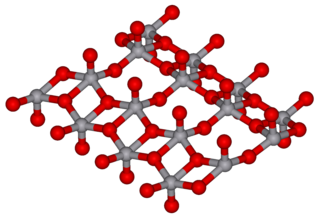
Vanadium(V) oxide (vanadia) is the inorganic compound with the formula V2O5. Commonly known as vanadium pentoxide, it is a brown/yellow solid, although when freshly precipitated from aqueous solution, its colour is deep orange. Because of its high oxidation state, it is both an amphoteric oxide and an oxidizing agent. From the industrial perspective, it is the most important compound of vanadium, being the principal precursor to alloys of vanadium and is a widely used industrial catalyst.

Antimony(III) oxide is the inorganic compound with the formula Sb2O3. It is the most important commercial compound of antimony. It is found in nature as the minerals valentinite and senarmontite. Like most polymeric oxides, Sb2O3 dissolves in aqueous solutions with hydrolysis. A mixed arsenic-antimony oxide occurs in nature as the very rare mineral stibioclaudetite.

Phosphorus pentoxide is a chemical compound with molecular formula P4O10 (with its common name derived from its empirical formula, P2O5). This white crystalline solid is the anhydride of phosphoric acid. It is a powerful desiccant and dehydrating agent.
In chemistry, an aluminate is a compound containing an oxyanion of aluminium, such as sodium aluminate. In the naming of inorganic compounds, it is a suffix that indicates a polyatomic anion with a central aluminium atom.

Bismuth(III) oxide is perhaps the most industrially important compound of bismuth. It is also a common starting point for bismuth chemistry. It is found naturally as the mineral bismite (monoclinic) and sphaerobismoite, but it is usually obtained as a by-product of the smelting of copper and lead ores. Dibismuth trioxide is commonly used to produce the "Dragon's eggs" effect in fireworks, as a replacement of red lead.

Curium(III) oxide is a compound composed of curium and oxygen with the chemical formula Cm2O3. It is a crystalline solid with a unit cell that contains two curium atoms and three oxygen atoms. The simplest synthesis equation involves the reaction of curium(III) metal with O2−: 2 Cm3+ + 3 O2− ---> Cm2O3. Curium trioxide can exist as five polymorphic forms. Two of the forms exist at extremely high temperatures, making it difficult for experimental studies to be done on the formation of their structures. The three other possible forms which curium sesquioxide can take are the body-centered cubic form, the monoclinic form, and the hexagonal form. Curium(III) oxide is either white or light tan in color and, while insoluble in water, is soluble in inorganic and mineral acids. Its synthesis was first recognized in 1955.
In chemical nomenclature, the IUPAC nomenclature of inorganic chemistry is a systematic method of naming inorganic chemical compounds, as recommended by the International Union of Pure and Applied Chemistry (IUPAC). It is published in Nomenclature of Inorganic Chemistry. Ideally, every inorganic compound should have a name from which an unambiguous formula can be determined. There is also an IUPAC nomenclature of organic chemistry.

Manganese(III) oxide is a chemical compound with the formula Mn2O3. It occurs in nature as the mineral bixbyite (recently changed to bixbyite-(Mn)) and is used in the production of ferrites and thermistors.
Aluminium(I) oxide is a compound of aluminium and oxygen with the chemical formula Al2O. It can be prepared by heating the stable oxide Al2O3 with elemental silicon at 1800 °C under vacuum.

Aluminium (or aluminum) combines characteristics of pre- and post-transition metals. Since it has few available electrons for metallic bonding, like its heavier group 13 congeners, it has the characteristic physical properties of a post-transition metal, with longer-than-expected interatomic distances. Furthermore, as Al3+ is a small and highly charged cation, it is strongly polarizing and aluminium compounds tend towards covalency; this behaviour is similar to that of beryllium (Be2+), an example of a diagonal relationship. However, unlike all other post-transition metals, the underlying core under aluminium's valence shell is that of the preceding noble gas, whereas for gallium and indium it is that of the preceding noble gas plus a filled d-subshell, and for thallium and nihonium it is that of the preceding noble gas plus filled d- and f-subshells. Hence, aluminium does not suffer the effects of incomplete shielding of valence electrons by inner electrons from the nucleus that its heavier congeners do. Aluminium's electropositive behavior, high affinity for oxygen, and highly negative standard electrode potential are all more similar to those of scandium, yttrium, lanthanum, and actinium, which have ds2 configurations of three valence electrons outside a noble gas core: aluminium is the most electropositive metal in its group. Aluminium also bears minor similarities to the metalloid boron in the same group; AlX3 compounds are valence isoelectronic to BX3 compounds (they have the same valence electronic structure), and both behave as Lewis acids and readily form adducts. Additionally, one of the main motifs of boron chemistry is regular icosahedral structures, and aluminium forms an important part of many icosahedral quasicrystal alloys, including the Al–Zn–Mg class.
Curium compounds are compounds containing the element curium (Cm). Curium usually forms compounds in the +3 oxidation state, although compounds with curium in the +4, +5 and +6 oxidation states are also known.
Protactinium compounds are compounds containing the element protactinium. These compounds usually have protactinium in the +5 oxidation state, although these compounds can also exist in the +2, +3 and +4 oxidation states.
References
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ Eyring, LeRoy; Holmberg, Bo (1963-01-01). "Ordered Phases and Nonstoichiometry in the Rare Earth Oxide System". Advances in Chemistry. Washington, D. C.: American Chemical Society. pp. 46–57. doi:10.1021/ba-1964-0039.ch004. ISBN 978-0-8412-0040-1. ISSN 0065-2393.
- ↑ Marezio, M. (1966-11-01). "Refinement of the crystal structure of In2O3 at two wavelengths". Acta Crystallographica. International Union of Crystallography (IUCr). 20 (6): 723–728. doi:10.1107/s0365110x66001749. ISSN 0365-110X.
- ↑ The Scrabble Omnibus, Gyles Brandreth, ISBN 0-00-218081-2
- ↑ , David K. Israel, "Scrabble Word Records", March 22, 2010, accessed March 31, 2018
- ↑ New York State, Legislature, Assembly (1860). Documents of the Assembly of the State of New York, Eighty-third Session. — 1860. Volume IV ; No. 111: Transactions of the State Medical Society, p. 19
- ↑ Keith W. Smith Total scrabble, page 67
- ↑ Record for the Highest Scoring Scrabble Move at scrabulizer.com, accessed 2008-05-30