Erbium is a chemical element; it has symbol Er and atomic number 68. A silvery-white solid metal when artificially isolated, natural erbium is always found in chemical combination with other elements. It is a lanthanide, a rare-earth element, originally found in the gadolinite mine in Ytterby, Sweden, which is the source of the element's name.
The lanthanide or lanthanoid series of chemical elements comprises at least the 14 metallic chemical elements with atomic numbers 57–70, from lanthanum through ytterbium. In the periodic table, they fill the 4f orbitals. Lutetium is also sometimes considered a lanthanide, despite being a d-block element and a transition metal.

Samarium is a chemical element; it has symbol Sm and atomic number 62. It is a moderately hard silvery metal that slowly oxidizes in air. Being a typical member of the lanthanide series, samarium usually has the oxidation state +3. Compounds of samarium(II) are also known, most notably the monoxide SmO, monochalcogenides SmS, SmSe and SmTe, as well as samarium(II) iodide.

Boron carbide (chemical formula approximately B4C) is an extremely hard boron–carbon ceramic, a covalent material used in tank armor, bulletproof vests, engine sabotage powders, as well as numerous industrial applications. With a Vickers hardness of >30 GPa, it is one of the hardest known materials, behind cubic boron nitride and diamond.

Hafnium diboride is a type of ceramic composed of hafnium and boron that belongs to the class of ultra-high temperature ceramics. It has a melting temperature of about 3250 °C. It is an unusual ceramic, having relatively high thermal and electrical conductivities, properties it shares with isostructural titanium diboride and zirconium diboride. It is a grey, metallic looking material. Hafnium diboride has a hexagonal crystal structure, a molar mass of 200.11 grams per mole, and a density of 11.2 g/cm3.

Erbium(III) chloride is a violet solid with the formula ErCl3. It is used in the preparation of erbium metal.
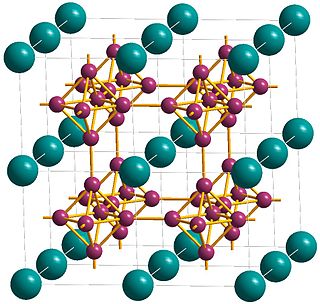
Lanthanum hexaboride (LaB6, also called lanthanum boride and LaB) is an inorganic chemical, a boride of lanthanum. It is a refractory ceramic material that has a melting point of 2210 °C, and is insoluble in water and hydrochloric acid. It is extremely hard, with a Mohs hardness of 9.5. It has a low work function and one of the highest electron emissivities known, and is stable in vacuum. Stoichiometric samples are colored intense purple-violet, while boron-rich ones (above LaB6.07) are blue. Ion bombardment changes its color from purple to emerald green. LaB6 is a superconductor with a relatively low transition temperature of 0.45 K.

Cerium hexaboride (CeB6, also called cerium boride, CeBix, CEBIX, and (incorrectly) CeB) is an inorganic chemical, a boride of cerium. It is a refractory ceramic material. It has a low work function, one of the highest electron emissivities known, and is stable in vacuum. The principal use of cerium hexaboride is a coating of hot cathodes. It usually operates at temperature of 1450 °C.

Strontium boride (SrB6) is an inorganic compound. At room temperature, it appears as a crystalline black powder. Closer examination reveals slightly translucent dark red crystals capable of scratching quartz. It is very stable and has a high melting point and density. Although not thought to be toxic, it is an irritant to the skin, eyes, and respiratory tract.
Samarium(III) sulfide (Sm2S3) is a chemical compound of the rare earth element samarium, and sulfur. In this compound samarium is in the +3 oxidation state, and sulfur is an anion in the −2 state.

Calcium hexaboride (sometimes calcium boride) is a compound of calcium and boron with the chemical formula CaB6. It is an important material due to its high electrical conductivity, hardness, chemical stability, and melting point. It is a black, lustrous, chemically inert powder with a low density. It has the cubic structure typical for metal hexaborides, with octahedral units of 6 boron atoms combined with calcium atoms. CaB6 and lanthanum-doped CaB6 both show weak ferromagnetic properties, which is a remarkable fact because calcium and boron are neither magnetic, nor have inner 3d or 4f electronic shells, which are usually required for ferromagnetism.

Zirconium diboride (ZrB2) is a highly covalent refractory ceramic material with a hexagonal crystal structure. ZrB2 is an ultra-high temperature ceramic (UHTC) with a melting point of 3246 °C. This along with its relatively low density of ~6.09 g/cm3 (measured density may be higher due to hafnium impurities) and good high temperature strength makes it a candidate for high temperature aerospace applications such as hypersonic flight or rocket propulsion systems. It is an unusual ceramic, having relatively high thermal and electrical conductivities, properties it shares with isostructural titanium diboride and hafnium diboride.

Yttrium boride refers to a crystalline material composed of different proportions of yttrium and boron, such as YB2, YB4, YB6, YB12, YB25, YB50 and YB66. They are all gray-colored, hard solids having high melting temperatures. The most common form is the yttrium hexaboride YB6. It exhibits superconductivity at relatively high temperature of 8.4 K and, similar to LaB6, is an electron cathode. Another remarkable yttrium boride is YB66. It has a large lattice constant (2.344 nm), high thermal and mechanical stability, and therefore is used as a diffraction grating for low-energy synchrotron radiation (1–2 keV).
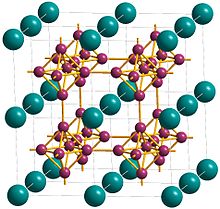
Metals, and specifically rare-earth elements, form numerous chemical complexes with boron. Their crystal structure and chemical bonding depend strongly on the metal element M and on its atomic ratio to boron. When B/M ratio exceeds 12, boron atoms form B12 icosahedra which are linked into a three-dimensional boron framework, and the metal atoms reside in the voids of this framework. Those icosahedra are basic structural units of most allotropes of boron and boron-rich rare-earth borides. In such borides, metal atoms donate electrons to the boron polyhedra, and thus these compounds are regarded as electron-deficient solids.

Samarium hexaboride (SmB6) is an intermediate-valence compound where samarium is present both as Sm2+ and Sm3+ ions at the ratio 3:7. It is a Kondo insulator having a metallic surface state.

Niobium diboride (NbB2) is a highly covalent refractory ceramic material with a hexagonal crystal structure.
Samarium compounds are compounds formed by the lanthanide metal samarium (Sm). In these compounds, samarium generally exhibits the +3 oxidation state, such as SmCl3, Sm(NO3)3 and Sm(C2O4)3. Compounds with samarium in the +2 oxidation state are also known, for example SmI2.

Promethium compounds are compounds containing the element promethium, which normally take the +3 oxidation state. Promethium belongs to the cerium group of lanthanides and is chemically very similar to the neighboring elements. Because of its instability, chemical studies of promethium are incomplete. Even though a few compounds have been synthesized, they are not fully studied; in general, they tend to be pink or red in color. Treatment of acidic solutions containing Pm3+ ions with ammonia results in a gelatinous light-brown sediment of hydroxide, Pm(OH)3, which is insoluble in water. When dissolved in hydrochloric acid, a water-soluble yellow salt, PmCl3, is produced; similarly, when dissolved in nitric acid, a nitrate results, Pm(NO3)3. The latter is also well-soluble; when dried, it forms pink crystals, similar to Nd(NO3)3. The electron configuration for Pm3+ is [Xe] 4f4, and the color of the ion is pink. The ground state term symbol is 5I4. The sulfate is slightly soluble, like the other cerium group sulfates. Cell parameters have been calculated for its octahydrate; they lead to conclusion that the density of Pm2(SO4)3·8 H2O is 2.86 g/cm3. The oxalate, Pm2(C2O4)3·10 H2O, has the lowest solubility of all lanthanide oxalates.
Erbium compounds are compounds containing the element erbium (Er). These compounds are usually dominated by erbium in the +3 oxidation state, although the +2, +1 and 0 oxidation states have also been reported.
Lanthanide compounds are compounds formed by the 15 elements classed as lanthanides. The lanthanides are generally trivalent, although some, such as cerium and europium, are capable of forming compounds in other oxidation states.