Potassium is the chemical element with the symbol K and atomic number 19. It is a silvery white metal that is soft enough to easily cut with a knife. Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure. It was first isolated from potash, the ashes of plants, from which its name derives. In the periodic table, potassium is one of the alkali metals, all of which have a single valence electron in the outer electron shell, which is easily removed to create an ion with a positive charge. In nature, potassium occurs only in ionic salts. Elemental potassium reacts vigorously with water, generating sufficient heat to ignite hydrogen emitted in the reaction, and burning with a lilac-colored flame. It is found dissolved in seawater, and occurs in many minerals such as orthoclase, a common constituent of granites and other igneous rocks.

Rhenium is a chemical element with the symbol Re and atomic number 75. It is a silvery-gray, heavy, third-row transition metal in group 7 of the periodic table. With an estimated average concentration of 1 part per billion (ppb), rhenium is one of the rarest elements in the Earth's crust. Rhenium has the third-highest melting point and second-highest boiling point of any element at 5869 K. Rhenium resembles manganese and technetium chemically and is mainly obtained as a by-product of the extraction and refinement of molybdenum and copper ores. Rhenium shows in its compounds a wide variety of oxidation states ranging from −1 to +7.

Potassium oxide (K2O) is an ionic compound of potassium and oxygen. It is a base. This pale yellow solid is the simplest oxide of potassium. It is a highly reactive compound that is rarely encountered. Some industrial materials, such as fertilizers and cements, are assayed assuming the percent composition that would be equivalent to K2O.

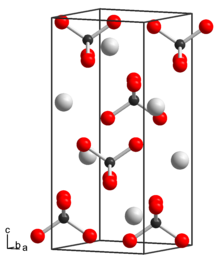
Potassium permanganate is an inorganic compound with the chemical formula KMnO4. It is a purplish-black crystalline salt, that dissolves in water as K+ and MnO−
4, an intensely pink to purple solution.
Cuprate loosely refers to a material that can be viewed as containing anionic copper complexes. Examples include tetrachloridocuprate ([CuCl4]2−), the superconductor YBa2Cu3O7, and the organocuprates (e.g., dimethylcuprate [Cu(CH3)2]−). The term cuprates derives from the Latin word for copper, cuprum. The term is mainly used in three contexts: oxide materials, anionic coordination complexes, and anionic organocopper compounds.

Perrhenic acid is the chemical compound with the formula Re2O7(H2O)2. It is obtained by evaporating aqueous solutions of Re2O7. Conventionally, perrhenic acid is considered to have the formula HReO4, and a species of this formula forms when rhenium(VII) oxide sublimes in the presence of water or steam. When a solution of Re2O7 is kept for a period of months, it breaks down and crystals of HReO4·H2O are formed, which contain tetrahedral ReO−4. For most purposes, perrhenic acid and rhenium(VII) oxide are used interchangeably. Rhenium can be dissolved in nitric or concentrated sulfuric acid to produce perrhenic acid.
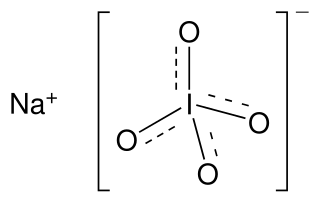
Sodium periodate is an inorganic salt, composed of a sodium cation and the periodate anion. It may also be regarded as the sodium salt of periodic acid. Like many periodates, it can exist in two different forms: sodium metaperiodate (formula NaIO4) and sodium orthoperiodate (normally Na2H3IO6, but sometimes the fully reacted salt Na5IO6). Both salts are useful oxidising agents.
Potassium cyanate is an inorganic compound with the formula KOCN. It is a colourless solid. It is used to prepare many other compounds including useful herbicide. Worldwide production of the potassium and sodium salts was 20,000 tons in 2006.
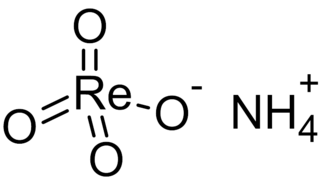
Ammonium perrhenate (APR) is the ammonium salt of perrhenic acid, NH4ReO4. It is the most common form in which rhenium is traded. It is a white salt; soluble in ethanol and water, and mildly soluble in NH4Cl. It was first described soon after the discovery of rhenium.
Selenium trioxide is the inorganic compound with the formula SeO3. It is white, hygroscopic solid. It is also an oxidizing agent and a Lewis acid. It is of academic interest as a precursor to Se(VI) compounds.

Sodium perrhenate (also known as sodium rhenate(VII)) is the inorganic compound with the formula NaReO4. It is a white salt that is soluble in water. It is a common precursor to other rhenium compounds. Its structure resembles that of sodium perchlorate and sodium permanganate.

In chemistry, the haloform reaction is a chemical reaction in which a haloform is produced by the exhaustive halogenation of an acetyl group, in the presence of a base. The reaction can be used to transform acetyl groups into carboxyl groups or to produce chloroform, bromoform, or iodoform. Note that fluoroform can't be prepared in this way.

Acetylenediol, or ethynediol, is a chemical substance with formula HO−C≡C−OH (an ynol). It is the diol of acetylene. Acetylenediol is unstable in the condensed phase, although its tautomer glyoxal (CHO)2 is well known.
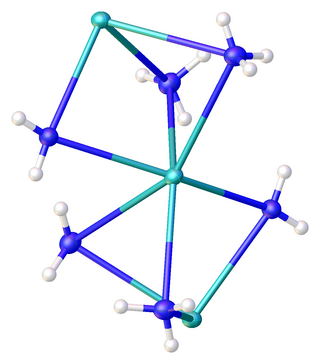
Potassium amide is an inorganic compound with the chemical formula KNH2. Like other alkali metal amides, it is a white solid that hydrolyzes readily. It is a strong base.

Antimony potassium tartrate, also known as potassium antimonyl tartrate, potassium antimontarterate, or tartar emetic, has the formula K2Sb2(C4H2O6)2. The compound has long been known as a powerful emetic, and was used in the treatment of schistosomiasis and leishmaniasis. It is used as a resolving agent. It typically is obtained as a hydrate.
Tetrafluoroberyllate or orthofluoroberyllateBeF2−
4 is an anion containing beryllium and fluorine. The fluoroanion has a tetrahedral shape, with the four fluorine atoms surrounding a central beryllium atom. It has the same size and outer electron structure as sulfate. Therefore, many compounds that contain sulfate have equivalents with tetrafluoroberyllate. Examples of these are the langbeinites, and Tutton's salts.
Rubidium hydrogen sulfate, sometimes referred to as rubidium bisulfate, is the half neutralized rubidium salt of sulfuric acid. It has the formula RbHSO4.
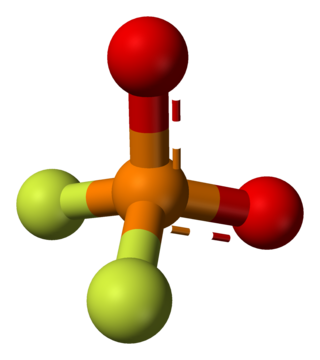
Difluorophosphate or difluorodioxophosphate or phosphorodifluoridate is an anion with formula PO
2F−
2. It has a single negative charge and resembles perchlorate (ClO−
4) and monofluorosulfonate (SO3F−) in shape and compounds. These ions are isoelectronic, along with tetrafluoroaluminate, phosphate, orthosilicate, and sulfate. It forms a series of compounds. The ion is toxic to mammals as it causes blockage to iodine uptake in the thyroid. However it is degraded in the body over several hours.

Rubidium sulfide is an inorganic compound and a salt with the chemical formula Rb2S. It is a white solid with similar properties to other alkali metal sulfides.
Rhenium compounds are compounds formed by the transition metal rhenium (Re). Rhenium can form in many oxidation states, and compounds are known for every oxidation state from -3 to +7 except -2, although the oxidation states +7, +6, +4, and +2 are the most common. Rhenium is most available commercially as salts of perrhenate, including sodium and ammonium perrhenates. These are white, water-soluble compounds. Tetrathioperrhenate anion [ReS4]− is possible.