Thallium(III) hydroxide, Tl(OH)3, also known as thallic hydroxide, is a hydroxide of thallium. It is a white solid.
Indium(III) sulfate (In2(SO4)3) is a sulfate salt of the metal indium. It is a sesquisulfate, meaning that the sulfate group occurs 11/2 times as much as the metal. It may be formed by the reaction of indium, its oxide, or its carbonate with sulfuric acid. An excess of strong acid is required, otherwise insoluble basic salts are formed. As a solid indium sulfate can be anhydrous, or take the form of a pentahydrate with five water molecules or a nonahydrate with nine molecules of water. Indium sulfate is used in the production of indium or indium containing substances. Indium sulfate also can be found in basic salts, acidic salts or double salts including indium alum.
Magnesium compounds are compounds formed by the element magnesium (Mg). These compounds are important to industry and biology, including magnesium carbonate, magnesium chloride, magnesium citrate, magnesium hydroxide, magnesium oxide, magnesium sulfate, and magnesium sulfate heptahydrate.

Scandium triiodide, also known as scandium iodide, is an inorganic compound with the formula ScI3 and is classified as a lanthanide iodide. This salt is a yellowish powder. It is used in metal halide lamps together with similar compounds, such as caesium iodide, because of their ability to maximize emission of UV and to prolong bulb life. The maximized UV emission can be tuned to a range that can initiate photopolymerizations.
Yttrium(III) sulfate is an inorganic compound with the formula Y2(SO4)3. The most common form is the anhydrate and octahydrate.

Europium dichloride is an inorganic compound with a chemical formula EuCl2. When it is irradiated by ultraviolet light, it has bright blue fluorescence.

Lutetium(III) hydroxide is an inorganic compound with the chemical formula Lu(OH)3.
Indium(III) nitrate is a nitrate salt of indium. It reacts with sodium tungstate to form In(OH)WO4, [In(OH)2]2WO4, NaInWO4 or In2(WO4)3 depending on pH.
Rhodium(III) hydroxide is a chemical compound with the formula Rh(OH)3.

Neodymium tantalate is an inorganic compound with the chemical formula NdTaO4. It is prepared by reacting neodymium oxide and tantalum pentoxide at 1200 °C. It reacts with a mixture of tantalum pentoxide and chlorine gas at high temperature to obtain Nd2Ta2O7Cl2. It is ammonolyzed at high temperature to obtain oxynitrides of Nd-Ta.

Neodymium perrhenate is an inorganic compound with the chemical formula Nd(ReO4)3, which exists in anhydrous and tetrahydrate. It can be obtained by reacting excess neodymium oxide with 240 g/L perrhenic acid solution. In its solution, NdReO42+ and Nd(ReO4)2+ can be observed with stability constants of 16.5 and 23.6, respectively.

Europium(III) acetate is an inorganic salt of europium and acetic acid with the chemical formula of Eu(CH3COO)3. In this compound, europium exhibits the +3 oxidation state. It can exist in the anhydrous form, sesquihydrate and tetrahydrate. Its hydrate molecule is a dimer.
Neodymium molybdate is an inorganic compound, with the chemical formula of Nd2(MoO4)3. It reacts with sodium molybdate at a high temperature to obtain NaNd(MoO4)2. It reacts at roughly 350°C to 700°C with hydrogen sulfide to obtain neodymium sulfide and molybdenum disulfide. At roughly 780K to 870K, it can be reduced by hydrogen to obtain Nd2Mo3O9.

Europium compounds are compounds formed by the lanthanide metal europium (Eu). In these compounds, europium generally exhibits the +3 oxidation state, such as EuCl3, Eu(NO3)3 and Eu(CH3COO)3. Compounds with europium in the +2 oxidation state are also known. The +2 ion of europium is the most stable divalent ion of lanthanide metals in aqueous solution. Lipophilic europium complexes often feature acetylacetonate-like ligands, e.g., Eufod.

Terbium compounds are compounds formed by the lanthanide metal terbium (Tb). Terbium generally exhibits the +3 oxidation state in these compounds, such as in TbCl3, Tb(NO3)3 and Tb(CH3COO)3. Compounds with terbium in the +4 oxidation state are also known, such as TbO2 and BaTbF6. Terbium can also form compounds in the 0, +1 and +2 oxidation states.

Promethium(III) phosphate is an inorganic compound, a salt of promethium and phosphate, with the chemical formula of PmPO4. It is radioactive. Its hydrate can be obtained by precipitation of soluble promethium salt and diammonium hydrogen phosphate at pH 3~4 (or obtained by hydrothermal reaction ), and the hydrate can be obtained by burning at 960 °C to obtain the anhydrous form. Its standard enthalpy of formation is −464 kcal/mol.

Neodymium(III) phosphate is an inorganic compound, with the chemical formula of NdPO4. Its hemihydrate can be obtained by the reaction of neodymium(III) chloride and phosphoric acid; its anhydrous form can be obtained by the reaction of silicon pyrophosphate (SiP2O7) and neodymium(III) fluoride. It reacts with calcium pyrophosphate to obtain Ca9Nd(PO4)7.

Sodium hexachloroiridate(III) is an inorganic compound with the chemical formula Na3IrCl6.
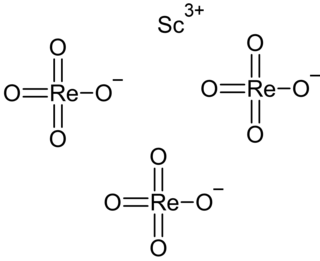
Scandium perrhenate is an inorganic compound, with the chemical formula Sc(ReO4)3. Its thermal stability is lower than that of the corresponding compounds of the yttrium and lanthanum perrhenates.
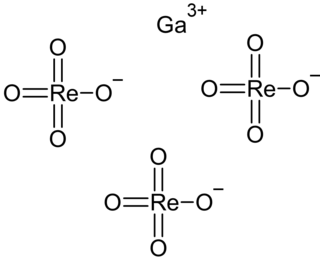
Gallium perrhenate is an inorganic compound with the chemical formula of Ga(ReO4)3. It exists in the anhydrous and hydrate forms.












