
Trichlorosilane (TCS) is an inorganic compound with the formula HCl3Si. It is a colourless, volatile liquid. Purified trichlorosilane is the principal precursor to ultrapure silicon in the semiconductor industry. In water, it rapidly decomposes to produce a siloxane polymer while giving off hydrochloric acid. Because of its reactivity and wide availability, it is frequently used in the synthesis of silicon-containing organic compounds.
In organic chemistry, an acyl chloride is an organic compound with the functional group −C(=O)Cl. Their formula is usually written R−COCl, where R is a side chain. They are reactive derivatives of carboxylic acids. A specific example of an acyl chloride is acetyl chloride, CH3COCl. Acyl chlorides are the most important subset of acyl halides.
Cyclopropene is an organic compound with the formula C3H4. It is the simplest cycloalkene. Because the ring is highly strained, cyclopropene is difficult to prepare and highly reactive. This colorless gas has been the subject for many fundamental studies of bonding and reactivity. It does not occur naturally, but derivatives are known in some fatty acids. Derivatives of cyclopropene are used commercially to control ripening of some fruit.
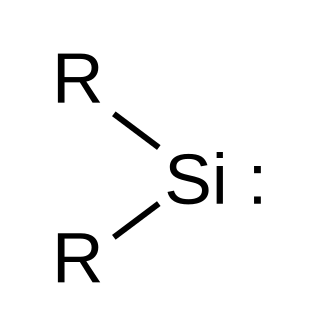
Silylene is a chemical compound with the formula SiR2. It is the silicon analog of carbene. Due to presence of a vacant p orbital, silylene rapidly reacts in a bimolecular manner when condensed. Unlike carbenes, which can exist in the singlet or triplet state, silylene (and all of its derivatives) are singlets.

Organotin chemistry is the scientific study of the synthesis and properties of organotin compounds or stannanes, which are organometallic compounds containing tin–carbon bonds. The first organotin compound was diethyltin diiodide, discovered by Edward Frankland in 1849. The area grew rapidly in the 1900s, especially after the discovery of the Grignard reagents, which are useful for producing Sn–C bonds. The area remains rich with many applications in industry and continuing activity in the research laboratory.

In organosilicon chemistry, a siloxane is an organic compound containing a functional group of two silicon atoms bound to an oxygen atom: Si−O−Si. The parent siloxanes include the oligomeric and polymeric hydrides with the formulae H[OSiH2]nOH and [OSiH2]n. Siloxanes also include branched compounds, the defining feature of which is that each pair of silicon centres is separated by one oxygen atom. The siloxane functional group forms the backbone of silicones [−R2Si−O−SiR2−]n, the premier example of which is polydimethylsiloxane (PDMS). The functional group R3SiO− is called siloxy. Siloxanes are manmade and have many commercial and industrial applications because of the compounds’ hydrophobicity, low thermal conductivity, and high flexibility.
In inorganic chemistry, chlorosilanes are a group of reactive, chlorine-containing chemical compounds, related to silane and used in many chemical processes. Each such chemical has at least one silicon-chlorine bond. Trichlorosilane is produced on the largest scale. The parent chlorosilane is silicon tetrachloride.
The Reed reaction is a chemical reaction that utilizes light to oxidize hydrocarbons to alkylsulfonyl chlorides. This reaction is employed in modifying polyethylene to give chlorosulfonated polyethylene (CSPE), which is noted for its toughness.

Trimethylsilyl chloride, also known as chlorotrimethylsilane is an organosilicon compound, with the formula (CH3)3SiCl, often abbreviated Me3SiCl or TMSCl. It is a colourless volatile liquid that is stable in the absence of water. It is widely used in organic chemistry.

Organosilicon chemistry is the study of organometallic compounds containing carbon–silicon bonds, to which they are called organosilicon compounds. Most organosilicon compounds are similar to the ordinary organic compounds, being colourless, flammable, hydrophobic, and stable to air. Silicon carbide is an inorganic compound.
In chemistry, dehydrohalogenation is an elimination reaction which removes a hydrogen halide from a substrate. The reaction is usually associated with the synthesis of alkenes, but it has wider applications.

Hexamethyldisiloxane (HMDSO or MM) is an organosilicon compound with the formula O[Si(CH3)3]2. This volatile colourless liquid is used as a solvent and as a reagent in organic synthesis. It is prepared by the hydrolysis of trimethylsilyl chloride. The molecule is the protypical disiloxane and resembles a subunit of polydimethylsiloxane.
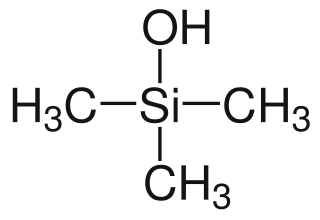
Trimethylsilanol (TMS) is an organosilicon compound with the formula (CH3)3SiOH. The Si centre bears three methyl groups and one hydroxyl group. It is a colourless volatile liquid.

Methyltrichlorosilane, also known as trichloromethylsilane, is a monomer and organosilicon compound with the formula CH3SiCl3. It is a colorless liquid with a sharp odor similar to that of hydrochloric acid. As methyltrichlorosilane is a reactive compound, it is mainly used a precursor for forming various cross-linked siloxane polymers.
The direct process, also called the direct synthesis, Rochow process, and Müller-Rochow process is the most common technology for preparing organosilicon compounds on an industrial scale. It was first reported independently by Eugene G. Rochow and Richard Müller in the 1940s.
In organosilicon chemistry, polysilazanes are polymers in which silicon and nitrogen atoms alternate to form the basic backbone. Since each silicon atom is bound to two separate nitrogen atoms and each nitrogen atom to two silicon atoms, both chains and rings of the formula [R2Si−NR]n occur. R can be hydrogen atoms or organic substituents. If all substituents R are hydrogen atoms, the polymer is designated as perhydropolysilazane, polyperhydridosilazane, or inorganic polysilazane ([H2Si−NH]n). If hydrocarbon substituents are bound to the silicon atoms, the polymers are designated as Organopolysilazanes. Molecularly, polysilazanes [R2Si−NH]n are isoelectronic with and close relatives to polysiloxanes [R2Si−O]n (silicones).

Polysilanes are organosilicon compounds with the formula (R2Si)n. They are relatives of traditional organic polymers but their backbones are composed of silicon atoms. They exhibit distinctive optical and electrical properties. They are mainly used as precursors to silicon carbide. The simplest polysilane would be (SiH2)n, which is mainly of theoretical, not practical interest.
In organosilicon chemistry, organosilanols are a group of chemical compounds derived from silicon. More specifically, they are carbosilanes derived with a hydroxy group on the silicon atom. Organosilanols are the silicon analogs to alcohols. Silanols are more acidic and more basic than their alcohol counterparts and therefore show a rich structural chemistry characterized by hydrogen bonding networks which are particularly well studied for silanetriols.
In organosilicon chemistry, silanes are a diverse class of charge-neutral organic compounds with the general formula SiR4. The R substituents can be any combination of organic or inorganic groups. Most silanes contain Si-C bonds, and are discussed under organosilicon compounds. Some contain Si-H bonds and are discussed under hydrosilanes.

Hexachlorodisiloxane is a chemical compound composed of chlorine, silicon, and oxygen. Structurally, it is the symmetrical ether of two trichlorosilyl groups, and can be synthesized via high-temperature oxidation of silicon tetrachloride:












![Container with the substance, showing UN number 1162, in Japan. Container [( 22T0 )] EXFU 175174(7) [( Pictures taken in Japan )] (cropped).jpg](http://upload.wikimedia.org/wikipedia/commons/thumb/7/70/Container_%E3%80%90_22T0_%E3%80%91_EXFU_175174%287%29_%E3%80%90_Pictures_taken_in_Japan_%E3%80%91_%28cropped%29.jpg/220px-Container_%E3%80%90_22T0_%E3%80%91_EXFU_175174%287%29_%E3%80%90_Pictures_taken_in_Japan_%E3%80%91_%28cropped%29.jpg)









