A Ziegler–Natta catalyst, named after Karl Ziegler and Giulio Natta, is a catalyst used in the synthesis of polymers of 1-alkenes (alpha-olefins). Two broad classes of Ziegler–Natta catalysts are employed, distinguished by their solubility:
A polyphosphate is a salt or ester of polymeric oxyanions formed from tetrahedral PO4 (phosphate) structural units linked together by sharing oxygen atoms. Polyphosphates can adopt linear or a cyclic ring structures. In biology, the polyphosphate esters ADP and ATP are involved in energy storage. A variety of polyphosphates find application in mineral sequestration in municipal waters, generally being present at 1 to 5 ppm. GTP, CTP, and UTP are also nucleotides important in the protein synthesis, lipid synthesis, and carbohydrate metabolism, respectively. Polyphosphates are also used as food additives, marked E452.
A diol is a chemical compound containing two hydroxyl groups. An aliphatic diol is also called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified.

In organic chemistry, an imide is a functional group consisting of two acyl groups bound to nitrogen. The compounds are structurally related to acid anhydrides, although imides are more resistant to hydrolysis. In terms of commercial applications, imides are best known as components of high-strength polymers, called polyimides. Inorganic imides are also known as solid state or gaseous compounds, and the imido group (=NH) can also act as a ligand.

Titanium tetrachloride is the inorganic compound with the formula TiCl4. It is an important intermediate in the production of titanium metal and the pigment titanium dioxide. TiCl4 is a volatile liquid. Upon contact with humid air, it forms thick clouds of titanium dioxide and hydrochloric acid, a reaction that was formerly exploited for use in smoke machines. It is sometimes referred to as “tickle” or “tickle 4”, as a phonetic representation of the symbols of its molecular formula.
Cuprates are a class of compounds that contain copper (Cu). They can be broadly categorized into two main types:

In organosilicon chemistry, a siloxane is an organic compound containing a functional group of two silicon atoms bound to an oxygen atom: Si−O−Si. The parent siloxanes include the oligomeric and polymeric hydrides with the formulae H[OSiH2]nOH and [OSiH2]n. Siloxanes also include branched compounds, the defining feature of which is that each pair of silicon centres is separated by one oxygen atom. The siloxane functional group forms the backbone of silicones [−R2Si−O−SiR2−]n, the premier example of which is polydimethylsiloxane (PDMS). The functional group R3SiO− is called siloxy. Siloxanes are manmade and have many commercial and industrial applications because of the compounds’ hydrophobicity, low thermal conductivity, and high flexibility.

Phosphorous acid is the compound described by the formula H3PO3. This acid is diprotic, not triprotic as might be suggested by this formula. Phosphorous acid is an intermediate in the preparation of other phosphorus compounds. Organic derivatives of phosphorous acid, compounds with the formula RPO3H2, are called phosphonic acids.

Ozonide is the polyatomic anion O−3. Cyclic organic compounds formed by the addition of ozone to an alkene are also called ozonides.

Tetrasulfur tetranitride is an inorganic compound with the formula S4N4. This gold-poppy coloured solid is the most important binary sulfur nitride, which are compounds that contain only the elements sulfur and nitrogen. It is a precursor to many S-N compounds and has attracted wide interest for its unusual structure and bonding.

Palladium(II) acetate is a chemical compound of palladium described by the formula [Pd(O2CCH3)2]n, abbreviated [Pd(OAc)2]n. It is more reactive than the analogous platinum compound. Depending on the value of n, the compound is soluble in many organic solvents and is commonly used as a catalyst for organic reactions.

Copper(I) iodide is the inorganic compound with the formula CuI. It is also known as cuprous iodide. It is useful in a variety of applications ranging from organic synthesis to cloud seeding.

The trispyrazolylborate ligand, abbreviated Tp−, is an anionic tridentate and tripodal ligand. Trispyrazolylborate refers specifically to the anion [HB(C3N2H3)3]−. However, the term can also be used to refer to derivatives having substituents on the pyrazolyl rings. This class of compounds belongs to the family of ligands called scorpionate ligands.
Dimethyldichlorosilane is a tetrahedral, organosilicon compound with the formula Si(CH3)2Cl2. At room temperature it is a colorless liquid that readily reacts with water to form both linear and cyclic Si-O chains. Dimethyldichlorosilane is made on an industrial scale as the principal precursor to dimethylsilicone and polysilane compounds.

Organozirconium chemistry is the science of exploring the properties, structure, and reactivity of organozirconium compounds, which are organometallic compounds containing chemical bonds between carbon and zirconium. Organozirconium compounds have been widely studied, in part because they are useful catalysts in Ziegler-Natta polymerization.

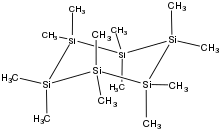
Polysilanes are organosilicon compounds with the formula (R2Si)n. They are relatives of traditional organic polymers but their backbones are composed of silicon atoms. They exhibit distinctive optical and electrical properties. They are mainly used as precursors to silicon carbide. The simplest polysilane would be (SiH2)n, which is mainly of theoretical, not practical interest.

Potassium selenocyanate is the inorganic compound with the formula KSeCN. It is a hygroscopic white solid that is soluble in water, decomposing in air to red selenium and potassium cyanide. The compound has been characterized by X-ray crystallography, which confirms that it is a salt. The C-N and C-Se distances are 112 and 183 pm, respectively consistent with triple and single bonds.

P4-t-Bu is a readily accessible chemical from the group of neutral, peralkylated sterically hindered polyaminophosphazenes, which are extremely strong bases but very weak nucleophiles, with the formula (CH3)3C−N=P(−N=P(−N(CH3)2)3)3. "t-Bu" stands for tert-butyl(CH3)3C–. "P4" stands for the fact that this molecule has 4 phosphorus atoms. P4-t-Bu can also be regarded as tetrameric triaminoiminophosphorane of the basic structure H−N=P(−NH2)3. The homologous series of P1 to P7 polyaminophosphazenes of the general formula with preferably methyl groups as R1, a methyl group or tert-butyl group as and even-numbered x between 0 and 6 (P4-t-Bu: R1 = Me, R2 = t-Bu and x = 3) has been developed by Reinhard Schwesinger; the resulting phosphazene bases are therefore also referred to as Schwesinger superbases.
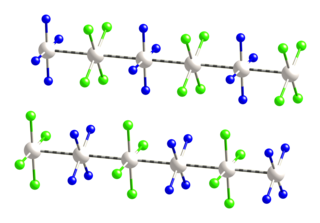
In chemistry and materials science, linear chain compounds are materials composed of one-dimensional arrays of metal-metal bonded molecules or ions. Such materials exhibit anisotropic electrical conductivity.

(Trimethylsilyl)methyllithium is classified both as an organolithium compound and an organosilicon compound. It has the empirical formula LiCH2Si(CH3)3, often abbreviated LiCH2tms. It crystallizes as the hexagonal prismatic hexamer [LiCH2tms]6, akin to some polymorphs of methyllithium. Many adducts have been characterized including the diethyl ether complexed cubane [Li4(μ3-CH2tms)4(Et2O)2] and [Li2(μ-CH2tms)2(tmeda)2].