A silanol is a functional group in silicon chemistry with the connectivity Si–O–H. It is related to the hydroxy functional group (C–O–H) found in all alcohols. Silanols are often invoked as intermediates in organosilicon chemistry and silicate mineralogy. If a silanol contains one or more organic residue, it is an organosilanol.

Tetrahedrane is a hypothetical platonic hydrocarbon with chemical formula C4H4 and a tetrahedral structure. The molecule would be subject to considerable angle strain and has not been synthesized as of 2021. However, a number of derivatives have been prepared. In a more general sense, the term tetrahedranes is used to describe a class of molecules and ions with related structure, e.g. white phosphorus.

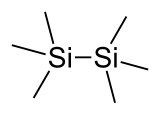
A trimethylsilyl group (abbreviated TMS) is a functional group in organic chemistry. This group consists of three methyl groups bonded to a silicon atom [−Si(CH3)3], which is in turn bonded to the rest of a molecule. This structural group is characterized by chemical inertness and a large molecular volume, which makes it useful in a number of applications.
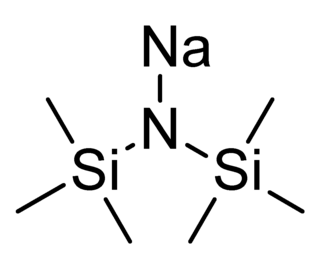
Sodium bis(trimethylsilyl)amide is the organosilicon compound with the formula NaN(Si 3)2. This species, usually called NaHMDS, is a strong base used for deprotonation reactions or base-catalyzed reactions. Its advantages are that it is commercially available as a solid and it is soluble not only in ethers, such as THF or diethyl ether, but also in aromatic solvents, like benzene and toluene by virtue of the lipophilic TMS groups.

Trimethylsilyl chloride, also known as chlorotrimethylsilane is an organosilicon compound (silyl halide), with the formula (CH3)3SiCl, often abbreviated Me3SiCl or TMSCl. It is a colourless volatile liquid that is stable in the absence of water. It is widely used in organic chemistry.

Organosilicon compounds are organometallic compounds containing carbon–silicon bonds. Organosilicon chemistry is the corresponding science of their preparation and properties. Most organosilicon compounds are similar to the ordinary organic compounds, being colourless, flammable, hydrophobic, and stable to air. Silicon carbide is an inorganic compound.

Bis(trimethylsilyl)mercury is a chemical reagent with the formula (CH3)3-Si-Hg-Si-(CH3)3.
Bis(trimethylsilyl)amine (also known as hexamethyldisilazane and HMDS) is an organosilicon compound with the molecular formula [(CH3)3Si]2NH. The molecule is a derivative of ammonia with trimethylsilyl groups in place of two hydrogen atoms. An electron diffraction study shows that silicon-nitrogen bond length (173.5 pm) and Si-N-Si bond angle (125.5°) to be similar to disilazane (in which methyl groups are replaced by hydrogen atoms) suggesting that steric factors are not a factor in regulating angles in this case. This colorless liquid is a reagent and a precursor to bases that are popular in organic synthesis and organometallic chemistry. Additionally, HMDS is also increasingly used as molecular precursor in chemical vapor deposition techniques to deposit silicon carbonitride thin films or coatings.

Hexamethyldisiloxane (HMDSO) is an organosilicon compound with the formula O[Si(CH3)3]2. This volatile colourless liquid is used as a solvent and as a reagent in organic synthesis. It is prepared by the hydrolysis of trimethylsilyl chloride. The molecule is the protypical disiloxane and resembles a subunit of polydimethylsiloxane.
Deoxygenation is a chemical reaction involving the removal of oxygen atoms from a molecule. The term also refers to the removal of molecular oxygen (O2) from gases and solvents, a step in air-free technique and gas purifiers. As applied to organic compounds, deoxygenation is a component of fuels production as well a type of reaction employed in organic synthesis, e.g. of pharmaceuticals.

Lithium bis(trimethylsilyl)amide is a lithiated organosilicon compound with the formula LiN(Si 3)2. It is commonly abbreviated as LiHMDS or Li(HMDS) and is primarily used as a strong non-nucleophilic base and as a ligand. Like many lithium reagents, it has a tendency to aggregate and will form a cyclic trimer in the absence of coordinating species.
The perrhenate ion is the anion with the formula ReO−
4, or a compound containing this ion. The perrhenate anion is tetrahedral, being similar in size and shape to perchlorate and the valence isoelectronic permanganate. The perrhenate anion is stable over a broad pH range and can be precipitated from solutions with the use of organic cations. At normal pH, perrhenate exists as metaperrhenate, but at high pH mesoperrhenate forms. Perrhenate, like its conjugate acid perrhenic acid, features rhenium in the oxidation state of +7 with a d0 configuration. Solid perrhenate salts takes on the color of the cation.
Silylation is the introduction of one or more (usually) substituted silyl groups (R3Si) to a molecule. The process is the basis of organosilicon chemistry.
Organosodium chemistry is the chemistry of organometallic compounds containing a carbon to sodium chemical bond. The application of organosodium compounds in chemistry is limited in part due to competition from organolithium compounds, which are commercially available and exhibit more convenient reactivity.

Metal bis(trimethylsilyl)amides are coordination complexes composed of a cationic metal with anionic bis(trimethylsilyl)amide ligands and are part of a broader category of metal amides.

Trimethylsilyl iodide (iodotrimethylsilane or TMSI) is an organosilicon compound with the chemical formula (CH3)3SiI. It is a colorless, volatile liquid at room temperature.

Trimethylsilyl cyclopentadiene is an organosilicon compound with the chemical formula C5H5Si(CH3)3. It exists as a colorless liquid. It is used in the synthesis of some metal cyclopentadienyl complexes and has attracted interest for its fluxional structure.
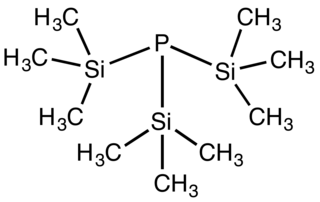
Tris(trimethylsilyl)phosphine is the organophosphorus compound with the formula P(SiMe3)3 (Me = methyl). It is a colorless liquid that ignites in air and hydrolyses readily.
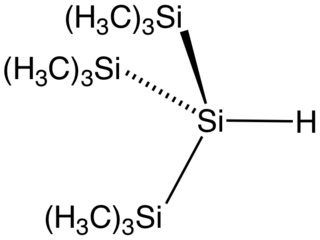
Tris(trimethylsilyl)silane is the organosilicon compound with the formula (Me3Si)3SiH (where Me = CH3). It is a colorless liquid that is classified as a hydrosilane since it contains an Si-H bond. The compound is notable as having a weak Si-H bond, with a bond dissociation energy estimated at 84 kcal/mol. For comparison, the Si-H bond in trimethylsilane is 94 kcal/mol. With such a weak bond, the compound is used as a reagent to deliver hydrogen atoms. The compound has been described as an environmentally benign analogue of tributyltin hydride.
In chemistry, transition metal silyl complexes describe coordination complexes in which a transition metal is bonded to an anionic silyl ligand, forming a metal-silicon sigma bond. This class of complexes are numerous and some are technologically significant as intermediates in hydrosilylation. These complexes are a subset of organosilicon compounds.