| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name Iodobenzene | |||
| Other names Phenyl iodide | |||
| Identifiers | |||
3D model (JSmol) | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.008.837 | ||
PubChem CID | |||
| UNII | |||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
| C6H5I | |||
| Molar mass | 204.01 g/mol | ||
| Appearance | colorless liquid | ||
| Density | 1.823 g/cm3 | ||
| Melting point | −29 °C (−20 °F; 244 K) | ||
| Boiling point | 188 °C (370 °F; 461 K) | ||
| Insoluble | |||
| log P | 3 | ||
| −92.00·10−6 cm3/mol | |||
| Viscosity | 1.5042 mPa·s (300.65 K) [1] | ||
| Hazards | |||
| Flash point | 74.44 °C (165.99 °F; 347.59 K) | ||
| Thermochemistry | |||
Heat capacity (C) | 0.779 J/K | ||
| Related compounds | |||
Related halobenzenes | Fluorobenzene Chlorobenzene Bromobenzene Astatobenzene | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
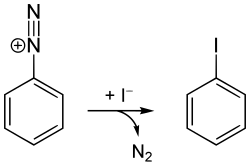
Iodobenzene is an aryl iodide and the simplest of the iodobenzenes, consisting of a benzene ring substituted with one iodine atom. Its chemical formula is C6H5I. It is useful as a synthetic intermediate in organic chemistry. It is a volatile colorless liquid, although aged samples appear yellowish.