Molecular biology is a branch of biology that seeks to understand the molecular basis of biological activity in and between cells, including biomolecular synthesis, modification, mechanisms, and interactions.

Polyacrylamide gel electrophoresis (PAGE) is a technique widely used in biochemistry, forensic chemistry, genetics, molecular biology and biotechnology to separate biological macromolecules, usually proteins or nucleic acids, according to their electrophoretic mobility. Electrophoretic mobility is a function of the length, conformation, and charge of the molecule. Polyacrylamide gel electrophoresis is a powerful tool used to analyze RNA samples. When polyacrylamide gel is denatured after electrophoresis, it provides information on the sample composition of the RNA species.

The western blot, or western blotting, is a widely used analytical technique in molecular biology and immunogenetics to detect specific proteins in a sample of tissue homogenate or extract. Besides detecting the proteins, this technique is also utilized to visualize, distinguish, and quantify the different proteins in a complicated protein combination.

HIV tests are used to detect the presence of the human immunodeficiency virus (HIV), the virus that causes HIV/AIDS, in serum, saliva, or urine. Such tests may detect antibodies, antigens, or RNA.

Staining is a technique used to enhance contrast in samples, generally at the microscopic level. Stains and dyes are frequently used in histology, in cytology, and in the medical fields of histopathology, hematology, and cytopathology that focus on the study and diagnoses of diseases at the microscopic level. Stains may be used to define biological tissues, cell populations, or organelles within individual cells.
A lysis buffer is a buffer solution used for the purpose of breaking open cells for use in molecular biology experiments that analyze the labile macromolecules of the cells. Most lysis buffers contain buffering salts and ionic salts to regulate the pH and osmolarity of the lysate. Sometimes detergents are added to break up membrane structures. For lysis buffers targeted at protein extraction, protease inhibitors are often included, and in difficult cases may be almost required. Lysis buffers can be used on both animal and plant tissue cells.

Protein electrophoresis is a method for analysing the proteins in a fluid or an extract. The electrophoresis may be performed with a small volume of sample in a number of alternative ways with or without a supporting medium, namely agarose or polyacrylamide. Variants of gel electrophoresis include SDS-PAGE, free-flow electrophoresis, electrofocusing, isotachophoresis, affinity electrophoresis, immunoelectrophoresis, counterelectrophoresis, and capillary electrophoresis. Each variant has many subtypes with individual advantages and limitations. Gel electrophoresis is often performed in combination with electroblotting or immunoblotting to give additional information about a specific protein.

In molecular biology and genetics, a blot is a method of transferring large biomolecules onto a carrier, such as a membrane composed of nitrocellulose, polyvinylidene fluoride or nylon. In many instances, this is done after a gel electrophoresis, transferring the molecules from the gel onto the blotting membrane, and other times adding the samples directly onto the membrane. After the blotting, the transferred molecules are then visualized by colorant staining, autoradiographic visualization of radiolabelled molecules, or specific labelling of some proteins or nucleic acids. The latter is done with antibodies or hybridization probes that bind only to some molecules of the blot and have an enzyme joined to them. After proper washing, this enzymatic activity is visualized by incubation with a proper reagent, rendering either a colored deposit on the blot or a chemiluminescent reaction which is registered by photographic film.
Coomassie brilliant blue is the name of two similar triphenylmethane dyes that were developed for use in the textile industry but are now commonly used for staining proteins in analytical biochemistry. Coomassie brilliant blue G-250 differs from Coomassie brilliant blue R-250 by the addition of two methyl groups. The name "Coomassie" is a registered trademark of Imperial Chemical Industries.
In pathology, silver staining is the use of silver to selectively alter the appearance of a target in microscopy of histological sections; in temperature gradient gel electrophoresis; and in polyacrylamide gels.
Protein methods are the techniques used to study proteins. There are experimental methods for studying proteins. Computational methods typically use computer programs to analyze proteins. However, many experimental methods require computational analysis of the raw data.

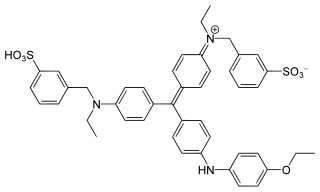
Ponceau S, Acid Red 112, or C.I. 27195 is a sodium salt of a diazo dye of a light red color, that may be used to prepare a stain for rapid reversible detection of protein bands on nitrocellulose or polyvinylidene fluoride (PVDF) membranes, as well as on cellulose acetate membranes. A Ponceau S stain is useful because it does not appear to have a deleterious effect on the sequencing of blotted polypeptides and is therefore one method of choice for locating polypeptides on western blots for blot-sequencing. It is also easily reversed with water washes, facilitating subsequent immunological detection. The stain can be completely removed from the protein bands by continued washing. Common stain formulations include 0.1% (w/v) Ponceau S in 5% acetic acid or 2% (w/v) Ponceau S in 30% trichloroacetic acid and 30% sulfosalicylic acid.
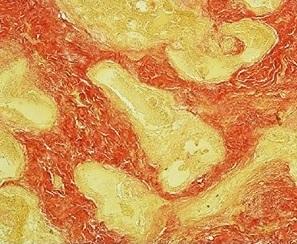
Van Gieson's stain is a mixture of picric acid and acid fuchsin. It is the simplest method of differential staining of collagen and other connective tissue. It was introduced to histology by American neuropsychiatrist and pathologist Ira Van Gieson.
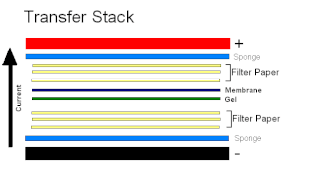
Electroblotting is a method in molecular biology/biochemistry/immunogenetics to transfer proteins or nucleic acids onto a membrane by using PVDF or nitrocellulose, after gel electrophoresis. The protein or nucleic acid can then be further analyzed using probes such as specific antibodies, ligands like lectins, or stains. This method can be used with all polyacrylamide and agarose gels. An alternative technique for transferring proteins from a gel is capillary blotting.
The eastern blot, or eastern blotting, is a biochemical technique used to analyze protein post-translational modifications including the addition of lipids, phosphates, and glycoconjugates. It is most often used to detect carbohydrate epitopes. Thus, eastern blot can be considered an extension of the biochemical technique of western blot. Multiple techniques have been described by the term "eastern blot(ting)", most use phosphoprotein blotted from sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gel on to a polyvinylidene fluoride or nitrocellulose membrane. Transferred proteins are analyzed for post-translational modifications using probes that may detect lipids, carbohydrate, phosphorylation or any other protein modification. Eastern blotting should be used to refer to methods that detect their targets through specific interaction of the post-translational modifications and the probe, distinguishing them from a standard far-western blot. In principle, eastern blotting is similar to lectin blotting.
The northwestern blot, also known as the northwestern assay, is a hybrid analytical technique of the western blot and the northern blot, and is used in molecular biology to detect interactions between RNA and proteins. A related technique, the western blot, is used to detect a protein of interest that involves transferring proteins that are separated by gel electrophoresis onto a nitrocellulose membrane. A colored precipitate clusters along the band on the membrane containing a particular target protein. A northern blot is a similar analytical technique that, instead of detecting a protein of interest, is used to study gene expression by detection of RNA on a similar membrane. The northwestern blot combines the two techniques, and specifically involves the identification of labeled RNA that interact with proteins that are immobilized on a similar nitrocellulose membrane.
An aldehyde tag is a short peptide tag that can be further modified to add fluorophores, glycans, PEG chains, or reactive groups for further synthesis. A short, genetically-encoded peptide with a consensus sequence LCxPxR is introduced into fusion proteins, and by subsequent treatment with the formylglycine-generating enzyme (FGE), the cysteine of the tag is converted to a reactive aldehyde group. This electrophilic group can be targeted by an array of aldehyde-specific reagents, such as aminooxy- or hydrazide-functionalized compounds.
A blotting matrix, in molecular biology and genetics, is the substrate onto which macromolecules, such as proteins, are transferred in a blot method. The matrices are generally chemically modified paper filters or microporous membrane filters. In a dot blot, macromolecules are applied directly to the matrix. Macromolecules can also be separated and transferred via gel electrophoresis.
Normalization of Western blot data is an analytical step that is performed to compare the relative abundance of a specific protein across the lanes of a blot or gel under diverse experimental treatments, or across tissues or developmental stages. The overall goal of normalization is to minimize effects arising from variations in experimental errors, such as inconsistent sample preparation, unequal sample loading across gel lanes, or uneven protein transfer, which can compromise the conclusions that can be obtained from Western blot data. Currently, there are two methods for normalizing Western blot data: (i) housekeeping protein normalization and (ii) total protein normalization.
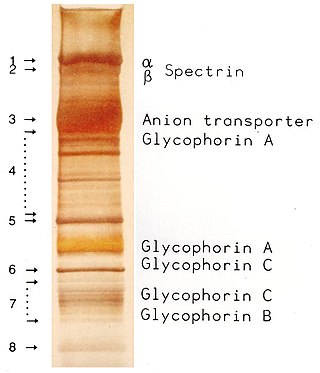
SDS-PAGE is a discontinuous electrophoretic system developed by Ulrich K. Laemmli which is commonly used as a method to separate proteins with molecular masses between 5 and 250 kDa. The combined use of sodium dodecyl sulfate and polyacrylamide gel eliminates the influence of structure and charge, and proteins are separated by differences in their size. At least up to 2012, the publication describing it was the most frequently cited paper by a single author, and the second most cited overall.













