 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.150.017 |
| Chemical and physical data | |
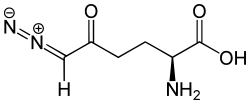
| Formula | C6H9N3O3 |
| Molar mass | 171.156 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
6-Diazo-5-oxo-L-norleucine (DON) is a glutamine antagonist, which was isolated originally from Streptomyces in a sample of Peruvian soil. This diazo compound is biosynthesized from lysine by three enzymes in bacteria. [2] It is one of the most famous non-proteinogenic amino acid and was characterized in 1956 by Henry W Dion et al., [3] who suggested a possible use in cancer therapy. This antitumoral efficacy was confirmed in different animal models. [4] DON was tested as chemotherapeutic agent in different clinical studies, but was never approved. In 2019, DON was shown to kill tumor cells while reversing disease symptoms and improve overall survival in late-stage experimental glioblastoma in mice, when combined with calorie-restricted ketogenic diet. [5]