
The citric acid cycle—also known as the Krebs cycle, Szent-Györgyi-Krebs cycle or the TCA cycle (tricarboxylic acid cycle)—is a series of biochemical reactions to release the energy stored in nutrients through the oxidation of acetyl-CoA derived from carbohydrates, fats, and proteins. The chemical energy released is available under the form of ATP. The Krebs cycle is used by organisms that respire (as opposed to organisms that ferment) to generate energy, either by anaerobic respiration or aerobic respiration. In addition, the cycle provides precursors of certain amino acids, as well as the reducing agent NADH, that are used in numerous other reactions. Its central importance to many biochemical pathways suggests that it was one of the earliest components of metabolism. Even though it is branded as a 'cycle', it is not necessary for metabolites to follow only one specific route; at least three alternative segments of the citric acid cycle have been recognized.

Glycolysis is the metabolic pathway that converts glucose into pyruvate and, in most organisms, occurs in the liquid part of cells. The free energy released in this process is used to form the high-energy molecules adenosine triphosphate (ATP) and reduced nicotinamide adenine dinucleotide (NADH). Glycolysis is a sequence of ten reactions catalyzed by enzymes.
A dehydrogenase is an enzyme belonging to the group of oxidoreductases that oxidizes a substrate by reducing an electron acceptor, usually NAD+/NADP+ or a flavin coenzyme such as FAD or FMN. Like all catalysts, they catalyze reverse as well as forward reactions, and in some cases this has physiological significance: for example, alcohol dehydrogenase catalyzes the oxidation of ethanol to acetaldehyde in animals, but in yeast it catalyzes the production of ethanol from acetaldehyde.

Acetyl-CoA is a molecule that participates in many biochemical reactions in protein, carbohydrate and lipid metabolism. Its main function is to deliver the acetyl group to the citric acid cycle to be oxidized for energy production.

Glutamate dehydrogenase is an enzyme observed in both prokaryotes and eukaryotic mitochondria. The aforementioned reaction also yields ammonia, which in eukaryotes is canonically processed as a substrate in the urea cycle. Typically, the α-ketoglutarate to glutamate reaction does not occur in mammals, as glutamate dehydrogenase equilibrium favours the production of ammonia and α-ketoglutarate. Glutamate dehydrogenase also has a very low affinity for ammonia, and therefore toxic levels of ammonia would have to be present in the body for the reverse reaction to proceed. However, in brain, the NAD+/NADH ratio in brain mitochondria encourages oxidative deamination. In bacteria, the ammonia is assimilated to amino acids via glutamate and aminotransferases. In plants, the enzyme can work in either direction depending on environment and stress. Transgenic plants expressing microbial GLDHs are improved in tolerance to herbicide, water deficit, and pathogen infections. They are more nutritionally valuable.

Oxaloacetic acid (also known as oxalacetic acid or OAA) is a crystalline organic compound with the chemical formula HO2CC(O)CH2CO2H. Oxaloacetic acid, in the form of its conjugate base oxaloacetate, is a metabolic intermediate in many processes that occur in animals. It takes part in gluconeogenesis, the urea cycle, the glyoxylate cycle, amino acid synthesis, fatty acid synthesis and the citric acid cycle.

Malate dehydrogenase (EC 1.1.1.37) (MDH) is an enzyme that reversibly catalyzes the oxidation of malate to oxaloacetate using the reduction of NAD+ to NADH. This reaction is part of many metabolic pathways, including the citric acid cycle. Other malate dehydrogenases, which have other EC numbers and catalyze other reactions oxidizing malate, have qualified names like malate dehydrogenase (NADP+).
In molecular biology, biosynthesis is a multi-step, enzyme-catalyzed process where substrates are converted into more complex products in living organisms. In biosynthesis, simple compounds are modified, converted into other compounds, or joined to form macromolecules. This process often consists of metabolic pathways. Some of these biosynthetic pathways are located within a single cellular organelle, while others involve enzymes that are located within multiple cellular organelles. Examples of these biosynthetic pathways include the production of lipid membrane components and nucleotides. Biosynthesis is usually synonymous with anabolism.

Isocitrate dehydrogenase (IDH) (EC 1.1.1.42) and (EC 1.1.1.41) is an enzyme that catalyzes the oxidative decarboxylation of isocitrate, producing alpha-ketoglutarate (α-ketoglutarate) and CO2. This is a two-step process, which involves oxidation of isocitrate (a secondary alcohol) to oxalosuccinate (a ketone), followed by the decarboxylation of the carboxyl group beta to the ketone, forming alpha-ketoglutarate. In humans, IDH exists in three isoforms: IDH3 catalyzes the third step of the citric acid cycle while converting NAD+ to NADH in the mitochondria. The isoforms IDH1 and IDH2 catalyze the same reaction outside the context of the citric acid cycle and use NADP+ as a cofactor instead of NAD+. They localize to the cytosol as well as the mitochondrion and peroxisome.

In the mitochondrion, the matrix is the space within the inner membrane. The word "matrix" stems from the fact that this space is viscous, compared to the relatively aqueous cytoplasm. The mitochondrial matrix contains the mitochondrial DNA, ribosomes, soluble enzymes, small organic molecules, nucleotide cofactors, and inorganic ions.[1] The enzymes in the matrix facilitate reactions responsible for the production of ATP, such as the citric acid cycle, oxidative phosphorylation, oxidation of pyruvate, and the beta oxidation of fatty acids.
The oxoglutarate dehydrogenase complex (OGDC) or α-ketoglutarate dehydrogenase complex is an enzyme complex, most commonly known for its role in the citric acid cycle.
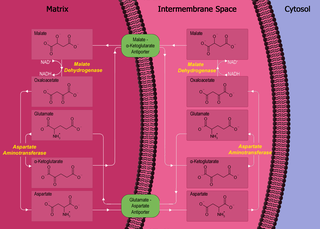
The malate–aspartate shuttle is a biochemical system for translocating electrons produced during glycolysis across the semipermeable inner membrane of the mitochondrion for oxidative phosphorylation in eukaryotes. These electrons enter the electron transport chain of the mitochondria via reduction equivalents to generate ATP. The shuttle system is required because the mitochondrial inner membrane is impermeable to NADH, the primary reducing equivalent of the electron transport chain. To circumvent this, malate carries the reducing equivalents across the membrane.

Amino acid biosynthesis is the set of biochemical processes by which the amino acids are produced. The substrates for these processes are various compounds in the organism's diet or growth media. Not all organisms are able to synthesize all amino acids. For example, humans can synthesize 11 of the 20 standard amino acids. These 11 are called the non-essential amino acids).
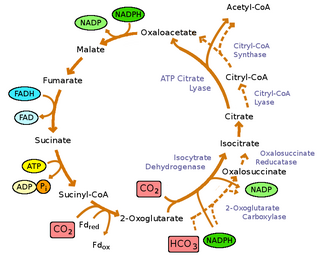
The reverse Krebs cycle is a sequence of chemical reactions that are used by some bacteria to produce carbon compounds from carbon dioxide and water by the use of energy-rich reducing agents as electron donors.
The mitochondrial shuttles are biochemical transport systems used to transport reducing agents across the inner mitochondrial membrane. NADH as well as NAD+ cannot cross the membrane, but it can reduce another molecule like FAD and [QH2] that can cross the membrane, so that its electrons can reach the electron transport chain.
In enzymology, a glutamine-pyruvate transaminase is an enzyme that catalyzes the chemical reaction

Aspartate aminotransferase, mitochondrial is an enzyme that in humans is encoded by the GOT2 gene. Glutamic-oxaloacetic transaminase is a pyridoxal phosphate-dependent enzyme which exists in cytoplasmic and inner-membrane mitochondrial forms, GOT1 and GOT2, respectively. GOT plays a role in amino acid metabolism and the urea and Kreb's cycle. Also, GOT2 is a major participant in the malate-aspartate shuttle, which is a passage from the cytosol to the mitochondria. The two enzymes are homodimeric and show close homology. GOT2 has been seen to have a role in cell proliferation, especially in terms of tumor growth.

Malate dehydrogenase, mitochondrial also known as malate dehydrogenase 2 is an enzyme that in humans is encoded by the MDH2 gene.
In biochemistry, the glutamate–glutamine cycle is a cyclic metabolic pathway which maintains an adequate supply of the neurotransmitter glutamate in the central nervous system. Neurons are unable to synthesize either the excitatory neurotransmitter glutamate, or the inhibitory GABA from glucose. Discoveries of glutamate and glutamine pools within intercellular compartments led to suggestions of the glutamate–glutamine cycle working between neurons and astrocytes. The glutamate/GABA–glutamine cycle is a metabolic pathway that describes the release of either glutamate or GABA from neurons which is then taken up into astrocytes. In return, astrocytes release glutamine to be taken up into neurons for use as a precursor to the synthesis of either glutamate or GABA.

The citrate-malate shuttle is a series of chemical reactions, commonly referred to as a biochemical cycle or system, that transports acetyl-CoA in the mitochondrial matrix across the inner and outer mitochondrial membranes for fatty acid synthesis. Mitochondria are enclosed in a double membrane. As the inner mitochondrial membrane is impermeable to acetyl-CoA, the shuttle system is essential to fatty acid synthesis in the cytosol. It plays an important role in the generation of lipids in the liver.















