In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group attached to an R-group. The general formula of a carboxylic acid is often written as R−COOH or R−CO2H, sometimes as R−C(O)OH with R referring to an organyl group, or hydrogen, or other groups. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion.
In chemistry, diamondoids are generalizations of the carbon cage molecule known as adamantane (C10H16), the smallest unit cage structure of the diamond crystal lattice. Diamondoids also known as nanodiamonds or condensed adamantanes may include one or more cages (adamantane, diamantane, triamantane, and higher polymantanes) as well as numerous isomeric and structural variants of adamantanes and polymantanes. These diamondoids occur naturally in petroleum deposits and have been extracted and purified into large pure crystals of polymantane molecules having more than a dozen adamantane cages per molecule. These species are of interest as molecular approximations of the diamond cubic framework, terminated with C−H bonds.

Hexamethylenetetramine, also known as methenamine, hexamine, or its trade name Urotropin, is a heterocyclic organic compound with the formula (CH2)6N4. This white crystalline compound is highly soluble in water and polar organic solvents. It has a cage-like structure similar to adamantane. It is useful in the synthesis of other organic compounds, including plastics, pharmaceuticals, and rubber additives. It sublimes in vacuum at 280 °C.
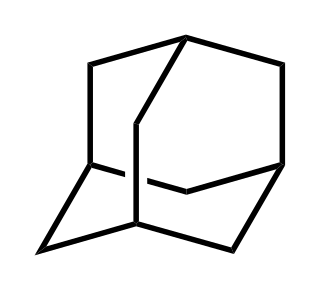
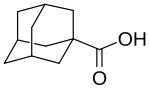
Adamantane is an organic compound with formula C10H16 or, more descriptively, (CH)4(CH2)6. Adamantane molecules can be described as the fusion of three cyclohexane rings. The molecule is both rigid and virtually stress-free. Adamantane is the most stable isomer of C10H16. The spatial arrangement of carbon atoms in the adamantane molecule is the same as in the diamond crystal. This similarity led to the name adamantane, which is derived from the Greek adamantinos (relating to steel or diamond). It is a white solid with a camphor-like odor. It is the simplest diamondoid.

Organotin chemistry is the scientific study of the synthesis and properties of organotin compounds or stannanes, which are organometallic compounds containing tin–carbon bonds. The first organotin compound was diethyltin diiodide, discovered by Edward Frankland in 1849. The area grew rapidly in the 1900s, especially after the discovery of the Grignard reagents, which are useful for producing Sn–C bonds. The area remains rich with many applications in industry and continuing activity in the research laboratory.

Phosphorus pentoxide is a chemical compound with molecular formula P4O10 (with its common name derived from its empirical formula, P2O5). This white crystalline solid is the anhydride of phosphoric acid. It is a powerful desiccant and dehydrating agent.

Palladium(II) acetate is a chemical compound of palladium described by the formula [Pd(O2CCH3)2]n, abbreviated [Pd(OAc)2]n. It is more reactive than the analogous platinum compound. Depending on the value of n, the compound is soluble in many organic solvents and is commonly used as a catalyst for organic reactions.

Chromium(II) acetate hydrate, also known as chromous acetate, is the coordination compound with the formula Cr2(CH3CO2)4(H2O)2. This formula is commonly abbreviated Cr2(OAc)4(H2O)2. This red-coloured compound features a quadruple bond. The preparation of chromous acetate once was a standard test of the synthetic skills of students due to its sensitivity to air and the dramatic colour changes that accompany its oxidation. It exists as the dihydrate and the anhydrous forms.
Ruthenium tetroxide is the inorganic compound with the formula RuO4. It is a yellow volatile solid that melts near room temperature. It has the odor of ozone. Samples are typically black due to impurities. The analogous OsO4 is more widely used and better known. It is also the anhydride of hyperruthenic acid (H2RuO5). One of the few solvents in which RuO4 forms stable solutions is CCl4.

Ammonium ferric citrate has the formula [NH+4]5[Fe(C6H4O7)2]5−. The iron in this compound is trivalent. All three carboxyl groups and the central hydroxyl group of citric acid are deprotonated. A distinguishing feature of this compound is that it is very soluble in water, in contrast to ferric citrate which is not very soluble.
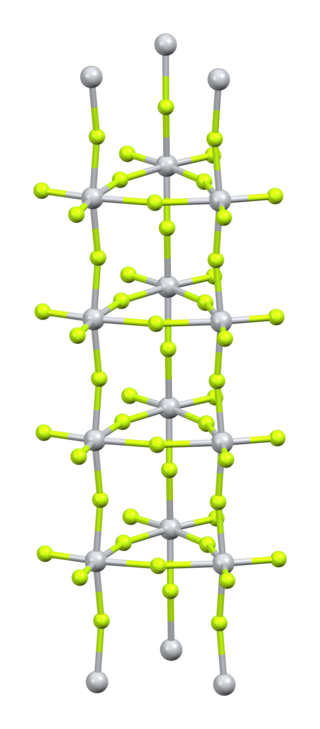
Titanium(IV) fluoride is the inorganic compound with the formula TiF4. It is a white hygroscopic solid. In contrast to the other tetrahalides of titanium, it adopts a polymeric structure. In common with the other tetrahalides, TiF4 is a strong Lewis acid.

N-Hydroxysuccinimide (NHS) is an organic compound with the formula (CH2CO)2NOH. It is a white solid that is used as a reagent for preparing active esters in peptide synthesis. It can be synthesized by heating succinic anhydride with hydroxylamine or hydroxylamine hydrochloride.
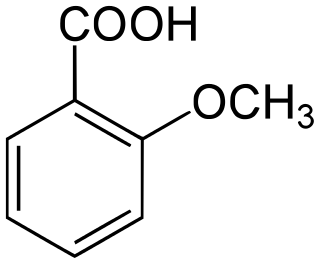
o-Anisic acid is an organic compound with the formula CH3OC6H4CO2H. A colorless solid, it is one of the isomers of anisic acid.

1,2-Diaminopropane (propane-1,2-diamine) is organic compound with the formula CH3CH(NH2)CH2NH2. A colorless liquid, it is the simplest chiral diamine. It is used as a bidentate ligand in coordination chemistry.

Diamantane is an organic compound that is a member of the diamondoids. These are cage hydrocarbons with structures similar to a subunit of the diamond lattice. It is a colorless solid that has been a topic of research since its discovery in oil and separation from deep natural gas condensates. Diamondoids such as diamantane exhibit unusual properties, including low surface energies, high densities, high hydrophobicities, and resistance to oxidation.

Thorium(IV) nitrate is a chemical compound, a salt of thorium and nitric acid with the formula Th(NO3)4. A white solid in its anhydrous form, it can form tetra- and pentahydrates. As a salt of thorium it is weakly radioactive.

Transition metal carboxylate complexes are coordination complexes with carboxylate (RCO2−) ligands. Reflecting the diversity of carboxylic acids, the inventory of metal carboxylates is large. Many are useful commercially, and many have attracted intense scholarly scrutiny. Carboxylates exhibit a variety of coordination modes, most common are κ1- (O-monodentate), κ2 (O,O-bidentate), and bridging.
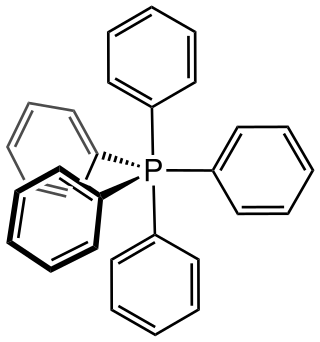
Pentaphenylphosphorus is an organic phosphorane containing five phenyl groups connected to a central phosphorus atom. The phosphorus atom is considered to be in the +5 oxidation state. The chemical formula could be written as P(C6H5)5 or Ph5P, where Ph represents the phenyl group. It was discovered and reported in 1949 by Georg Wittig.
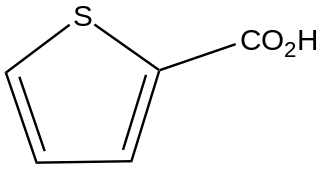
Thiophene-2-carboxylic acid is an organic compound with the formula SC4H3CO2H. It is one of two monocarboxylic acids of thiophene, the other being thiophene-3-carboxylic acid. Copper(I) thiophene-2-carboxylate is a catalyst for Ullmann coupling reactions.
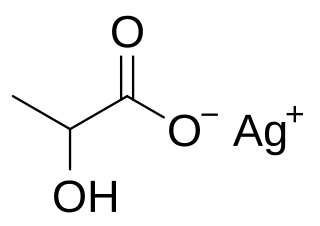
Silver lactate is an organic chemical compound, a salt of silver and lactic acid with the formula CH3CH(OH)COOAg.