
Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one methine group (=CH−) replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid with a distinctive, unpleasant fish-like smell. Pyridine is colorless, but older or impure samples can appear yellow, due to the formation of extended, unsaturated polymeric chains, which show significant electrical conductivity. The pyridine ring occurs in many important compounds, including agrochemicals, pharmaceuticals, and vitamins. Historically, pyridine was produced from coal tar. As of 2016, it is synthesized on the scale of about 20,000 tons per year worldwide.
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4H4NH. It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., N-methylpyrrole, C4H4NCH3. Porphobilinogen, a trisubstituted pyrrole, is the biosynthetic precursor to many natural products such as heme.
Hydantoin, or glycolylurea, is a heterocyclic organic compound with the formula CH2C(O)NHC(O)NH. It is a colorless solid that arises from the reaction of glycolic acid and urea. It is an oxidized derivative of imidazolidine. In a more general sense, hydantoins can refer to a groups and a class of compounds with the same ring structure as the parent. For example, phenytoin (mentioned below) has two phenyl groups substituted onto the number 5 carbon in a hydantoin molecule.

Benzimidazole is a heterocyclic aromatic organic compound. This bicyclic compound may be viewed as fused rings of the aromatic compounds benzene and imidazole. It is a white solid that appears in form of tabular crystals.

In organic chemistry, an aminal or aminoacetal is a functional group or type of organic compound that has two amine groups attached to the same carbon atom: −C(NR2)(NR2)−.. A common aminal is bis(dimethylamino)methane, a colorless liquid that is prepared by the reaction of dimethylamine and formaldehyde:
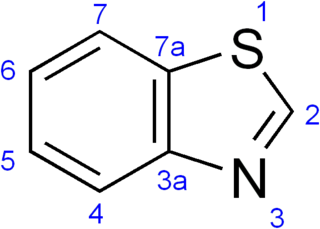
Benzothiazole is an aromatic heterocyclic compound with the chemical formula C
7H
5NS. It is colorless, slightly viscous liquid. Although the parent compound, benzothiazole is not widely used, many of its derivatives are found in commercial products or in nature. Firefly luciferin can be considered a derivative of benzothiazole.
Thiazolidine is a heterocyclic organic compound with the formula (CH2)3(NH)S. It is a 5-membered saturated ring with a thioether group and an amine group in the 1 and 3 positions. It is a sulfur analog of oxazolidine. Thiazolidine is a colorless liquid.

o-Phenylenediamine (OPD) is an organic compound with the formula C6H4(NH2)2. This aromatic diamine is an important precursor to many heterocyclic compounds. It is isomeric with m-phenylenediamine and p-phenylenediamine.

A persistent carbene (also known as stable carbene) is a type of carbene demonstrating particular stability. The best-known examples and by far largest subgroup are the N-heterocyclic carbenes (NHC) (sometimes called Arduengo carbenes), for example diaminocarbenes with the general formula (R2N)2C:, where the four R moieties are typically alkyl and aryl groups. The groups can be linked to give heterocyclic carbenes, such as those derived from imidazole, imidazoline, thiazole or triazole.
Pyrylium is a cation with formula C5H5O+, consisting of a six-membered ring of five carbon atoms, each with one hydrogen atom, and one positively charged oxygen atom. The bonds in the ring are conjugated as in benzene, giving it an aromatic character. In particular, because of the positive charge, the oxygen atom is trivalent. Pyrilium is a mono-cyclic and heterocyclic compound, one of the oxonium ions.

Imidazolidine is a heterocyclic compound (CH2)2(NH)2CH2. The parent imidazolidine is lightly studied, but related compounds substituted on one or both nitrogen centers are more common. Generally, they are colorless, polar, basic compounds. Imidazolidines are cyclic 5-membered examples of the general class of aminals.
Bis(trimethylsilyl)amine (also known as hexamethyldisilazane and HMDS) is an organosilicon compound with the molecular formula [(CH3)3Si]2NH. The molecule is a derivative of ammonia with trimethylsilyl groups in place of two hydrogen atoms. An electron diffraction study shows that silicon-nitrogen bond length (173.5 pm) and Si-N-Si bond angle (125.5°) to be similar to disilazane (in which methyl groups are replaced by hydrogen atoms) suggesting that steric factors are not a factor in regulating angles in this case. This colorless liquid is a reagent and a precursor to bases that are popular in organic synthesis and organometallic chemistry. Additionally, HMDS is also increasingly used as molecular precursor in chemical vapor deposition techniques to deposit silicon carbonitride thin films or coatings.

Imidazol-4-one-5-propionic acid is an intermediate in the metabolism of histidine. It is a colorless compound that is sensitive to light in air. The compound features an imidazolone ring.

2,4,6-Trimethylaniline is an organic compound with formula (CH3)3C6H2NH2. It is an aromatic amine that is of commercial interest as a precursor to dyes. It is prepared by selective mononitration of mesitylene, avoiding oxidation of the methyl groups. The resulting nitro compound is reduced to the aniline.

In chemistry, melem is a compound with formula C
6N
10H
6; specifically, 2,5,8-triamino-heptazine or 2,5,8-triamino-tri-s-triazine, whose molecule can be described as that of heptazine with the three hydrogen atoms replaced by amino groups. It is a white crystalline solid.

1-Methylimidazole or N-methylimidazole is an aromatic heterocyclic organic compound with the formula CH3C3H3N2. It is a colourless liquid that is used as a specialty solvent, a base, and as a precursor to some ionic liquids. It is a fundamental nitrogen heterocycle and as such mimics for various nucleoside bases as well as histidine and histamine.
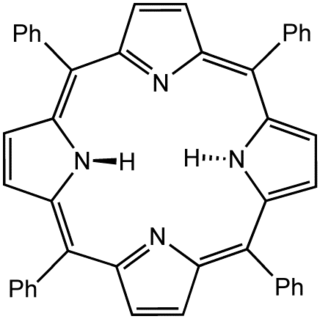
Tetraphenylporphyrin, abbreviated TPP or H2TPP, is a synthetic heterocyclic compound that resembles naturally occurring porphyrins. Porphyrins are dyes and cofactors found in hemoglobin and cytochromes and are related to chlorophyll and vitamin B12. The study of naturally occurring porphyrins is complicated by their low symmetry and the presence of polar substituents. Tetraphenylporphyrin is hydrophobic, symmetrically substituted, and easily synthesized. The compound is a dark purple solid that dissolves in nonpolar organic solvents such as chloroform and benzene.

In chemistry, hexahydro-1,3,5-triazine is a class of heterocyclic compounds with the formula (CH2NR)3. They are reduced derivatives of 1,3,5-triazine, which have the formula (CHN)3, a family of aromatic heterocycles. They are often called triazacyclohexanes or TACH's but this acronym is also applied to cis,cis-1,3,5-triaminocyclohexane.
A ring forming reaction or ring-closing reaction in organic chemistry is a general term for a variety of reactions that introduce one or more rings into a molecule. A heterocycle forming reaction is such a reaction that introduces a new heterocycle. Important classes of ring forming reactions include annulations and cycloadditions.

Imidazolidinones or imidazolinones are a class of 5-membered ring heterocycles structurally related to imidazole. Imidazolidinones feature a saturated C3N2 backbones, except for the presence of a urea or amide functional group in the 2 or 4 positions.














