GABA is the chief inhibitory neurotransmitter in the developmentally mature mammalian central nervous system. Its principal role is reducing neuronal excitability throughout the nervous system.

Phenelzine, sold under the brand name Nardil among others, is a non-selective and irreversible monoamine oxidase inhibitor (MAOI) of the hydrazine family which is primarily used as an antidepressant and anxiolytic to treat depression and anxiety. Along with tranylcypromine and isocarboxazid, phenelzine is one of the few non-selective and irreversible MAOIs still in widespread clinical use.

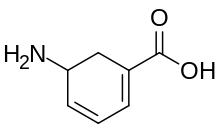
Vigabatrin, sold under the brand name Sabril among others, is a medication used in the management and treatment of infantile spasms and refractory complex partial seizures.

Succinic semialdehyde dehydrogenase deficiency (SSADHD) is a rare autosomal recessive disorder of the degradation pathway of the inhibitory neurotransmitter γ-aminobutyric acid, or GABA. The disorder has been identified in approximately 350 families, with a significant proportion being consanguineous families. The first case was identified in 1981 and published in a Dutch clinical chemistry journal that highlighted a number of neurological conditions such as delayed intellectual, motor, speech, and language as the most common manifestations. Later cases reported in the early 1990s began to show that hypotonia, hyporeflexia, seizures, and a nonprogressive ataxia were frequent clinical features as well.

A GABA receptor agonist is a drug that is an agonist for one or more of the GABA receptors, producing typically sedative effects, and may also cause other effects such as anxiolytic, anticonvulsant, and muscle relaxant effects. There are three receptors of the gamma-aminobutyric acid. The two receptors GABA-α and GABA-ρ are ion channels that are permeable to chloride ions which reduces neuronal excitability. The GABA-β receptor belongs to the class of G-Protein coupled receptors that inhibit adenylyl cyclase, therefore leading to decreased cyclic adenosine monophosphate (cAMP). GABA-α and GABA-ρ receptors produce sedative and hypnotic effects and have anti-convulsion properties. GABA-β receptors also produce similar effects. Furthermore, they lead to changes in gene transcription, and are mainly found in autonomic nervous system centers.

In enzymology, 4-aminobutyrate transaminase, also called GABA transaminase or 4-aminobutyrate aminotransferase, or GABA-T, is an enzyme that catalyzes the chemical reaction:

Dimethocaine, also known as DMC or larocaine, is a compound with a stimulatory effect. This effect resembles that of cocaine, although dimethocaine appears to be less potent. Just like cocaine, dimethocaine is addictive due to its stimulation of the reward pathway in the brain. However, dimethocaine is a legal cocaine replacement in some countries and is even listed by the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) under the category “synthetic cocaine derivatives”. The structure of dimethocaine, being a 4-aminobenzoic acid ester, resembles that of procaine. It is found as a white powder at room temperature.
A convulsant is a drug which induces convulsions and/or epileptic seizures, the opposite of an anticonvulsant. These drugs generally act as stimulants at low doses, but are not used for this purpose due to the risk of convulsions and consequent excitotoxicity. Most convulsants are antagonists at either the GABAA or glycine receptors, or ionotropic glutamate receptor agonists. Many other drugs may cause convulsions as a side effect at high doses but only drugs whose primary action is to cause convulsions are known as convulsants. Nerve agents such as sarin, which were developed as chemical weapons, produce convulsions as a major part of their toxidrome, but also produce a number of other effects in the body and are usually classified separately. Dieldrin which was developed as an insecticide blocks chloride influx into the neurons causing hyperexcitability of the CNS and convulsions. The Irwin observation test and other studies that record clinical signs are used to test the potential for a drug to induce convulsions. Camphor, and other terpenes given to children with colds can act as convulsants in children who have had febrile seizures.

para-Chloroamphetamine (PCA), also known as 4-chloroamphetamine (4-CA), is a serotonin–norepinephrine–dopamine releasing agent (SNDRA) and serotonergic neurotoxin of the amphetamine family. It is used in scientific research in the study of the serotonin system, as a serotonin releasing agent (SRA) at lower doses to produce serotonergic effects, and as a serotonergic neurotoxin at higher doses to produce long-lasting depletions of serotonin.
In pharmacology, a GABA transaminase inhibitor is an enzyme inhibitor that acts upon GABA transaminase. Inhibition of GABA transaminase enzymes reduces the degradation of GABA, leading to increased neuronal GABA concentrations.

Ethanolamine-O-sulfate (EOS) is an ester of sulfuric acid and ethanolamine. EOS is a GABA transaminase inhibitor which prevents the metabolism of GABA. It is used as a biochemical tool in studies involving GABA.
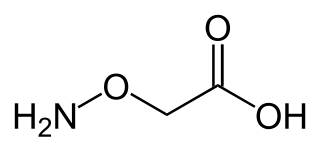
Aminooxyacetic acid, often abbreviated AOA or AOAA, is a compound that inhibits 4-aminobutyrate aminotransferase (GABA-T) activity in vitro and in vivo, leading to less gamma-aminobutyric acid (GABA) being broken down. Subsequently, the level of GABA is increased in tissues. At concentrations high enough to fully inhibit 4-aminobutyrate aminotransferase activity, aminooxyacetic acid is indicated as a useful tool to study regional GABA turnover in rats.
4-aminobutyrate---pyruvate transaminase is an enzyme with systematic name 4-aminobutanoate:pyruvate aminotransferase. This enzyme is a type of GABA transaminase, which degrades the neurotransmitter GABA. The enzyme catalyses the following chemical reaction

CI-966 (developmental code name) is a central nervous system depressant acting as a GABA reuptake inhibitor, specifically a highly potent and selective blocker of the GABA transporter 1 (GAT-1) (IC50 = 0.26 μM), and hence indirect and non-selective GABA receptor full agonist. It was investigated as a potential anticonvulsant, anxiolytic, and neuroprotective therapeutic but was discontinued during clinical development due to the incidence of severe adverse effects at higher doses and hence was never marketed.

4-Aminobutyrate aminotransferase is a protein that in humans is encoded by the ABAT gene. This gene is located in chromosome 16 at position of 13.2. This gene goes by a number of names, including, GABA transaminase, GABAT, 4-aminobutyrate transaminase, NPD009 etc. This gene is mainly and abundant located in neuronal tissues. 4-Aminobutyrate aminotransferase belongs to group of pyridoxal 5-phosphate-dependent enzyme which activates a large portion giving reaction to amino acids. ABAT is made up of two monomers of enzymes where each subunit has a molecular weight of 50kDa. It is identified that almost tierce of human synapses have GABA. GABA is a neurotransmitter that has different roles in different regions of the central and peripheral nervous systems. It can be found also in some tissues that do not have neurons. In addition, GAD and GABA-AT are responsible in regulating the concentration of GABA.

Dioscorine is an alkaloid toxin isolated from the tubers of tropical yam on several continents. It has been used as a monkey poison in some African countries, and as an arrow poison to aid in hunting in several parts of Asia. It was first isolated from Dioscorea hirsute by Boorsma in 1894 and obtained in a crystalline form by Schutte in 1897, and has since been found in other Dioscorea species. Dioscorine is a neurotoxin that acts by blocking the nicotinic acetylcholine receptor. Dioscorine is generally isolated in tandem with other alkaloids such as dioscin but is usually the most potent toxin in the mixture. It is a convulsant, producing symptoms similar to picrotoxin, with which it shares a similar mechanism of action.

IPTBO is a bicyclic phosphate convulsant. It is an extremely potent GABA receptor antagonist that can cause violent convulsions in mice.

Guanitoxin (GNT), formerly known as anatoxin-a(S) "Salivary", is a naturally occurring cyanotoxin commonly isolated from cyanobacteria. It is a potent covalent acetylcholinesterase inhibitor, and thus a potent rapid acting neurotoxin which in cases of severe exposure can lead to death. Guanitoxin was first structurally characterized in 1989, and consists of a cyclic N-hydroxyguanidine organophosphate with a phosphate ester moiety.

2,4-Diaminobutyric acid, also known as DABA, is GABA-T non-competitive inhibitor and a GABA reuptake inhibitor.

γ-Acetylenic GABA, also known as 4-amino-5-hexynoic acid, is a potent and irreversible inhibitor of GABA-T.