Omega−3 fatty acids, also called Omega−3 oils, ω−3 fatty acids, Ω-3 Fatty acids or n−3 fatty acids, are polyunsaturated fatty acids (PUFAs) characterized by the presence of a double bond three atoms away from the terminal methyl group in their chemical structure. They are widely distributed in nature, being important constituents of animal lipid metabolism, and they play an important role in the human diet and in human physiology. The three types of omega−3 fatty acids involved in human physiology are α-linolenic acid (ALA), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). ALA can be found in plants, while DHA and EPA are found in algae and fish. Marine algae and phytoplankton are primary sources of omega−3 fatty acids. DHA and EPA accumulate in fish that eat these algae. Common sources of plant oils containing ALA include walnuts, edible seeds, and flaxseeds as well as hempseed oil, while sources of EPA and DHA include fish and fish oils, and algae oil.
Essential fatty acids, or EFAs, are fatty acids that are required by humans and other animals for normal physiological function that cannot be synthesized in the body–either at all or in sufficient quantities – and thus must be obtained from a dietary source. Essential nutrients are indispensable for various cellular metabolic processes and for the maintenance and function of tissues and organs.

α-Linolenic acid, also known as alpha-linolenic acid (ALA), is an n−3, or omega-3, essential fatty acid. ALA is found in many seeds and oils, including flaxseed, walnuts, chia, hemp, and many common vegetable oils.

Krill oil is an extract prepared from a species of Antarctic krill, Euphausia superba. Processed krill oil is commonly sold as a dietary supplement. Two components of krill oil are omega-3 fatty acids similar to those in fish oil, and phospholipid-derived fatty acids (PLFA), mainly phosphatidylcholine. Fishing for krill where previously the focus was on marine life of higher trophic level is an example of fishing down the food web.
Fish oil is oil derived from the tissues of oily fish. Fish oils contain the omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), precursors of certain eicosanoids that are known to reduce inflammation in the body and improve hypertriglyceridemia. There has been a great deal of controversy in the 21st century about the role of fish oil in cardiovascular disease, with recent meta-analyses reaching different conclusions about its potential impact.

Vegetarian nutrition is the set of health-related challenges and advantages of vegetarian diets.
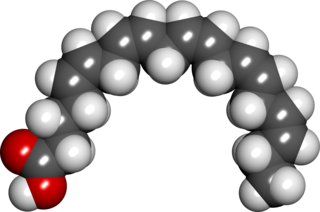
Eicosapentaenoic acid is an omega-3 fatty acid. In physiological literature, it is given the name 20:5(n-3). It also has the trivial name timnodonic acid. In chemical structure, EPA is a carboxylic acid with a 20-carbon chain and five cis double bonds; the first double bond is located at the third carbon from the omega end.
Dihomo-γ-linolenic acid (DGLA) is a 20-carbon ω−6 fatty acid. In physiological literature, it is given the name 20:3 (ω−6). DGLA is a carboxylic acid with a 20-carbon chain and three cis double bonds; the first double bond is located at the sixth carbon from the omega end. DGLA is the elongation product of γ-linolenic acid. GLA, in turn, is a desaturation product of linoleic acid. DGLA is made in the body by the elongation of GLA, by an efficient enzyme which does not appear to suffer any form of (dietary) inhibition. DGLA is an extremely uncommon fatty acid, found only in trace amounts in animal products.
Fatty acid desaturases are a family of enzymes that convert saturated fatty acids into unsaturated fatty acids and polyunsaturated fatty acids. For the common fatty acids of the C18 variety, desaturases convert stearic acid into oleic acid. Other desaturases convert oleic acid into linoleic acid, which is the precursor to alpha-linolenic acid, gamma-linolenic acid, and eicosatrienoic acid.
Eicosatetraenoic acid (ETA) designates any straight chain tetra-unsaturated 20-carbon fatty acid. The pure compounds, which are rarely encountered, are colorless oils. Two isomers, both of them essential fatty acids, are of particular interest:
There is a wide variety of fatty acids found in nature. Two classes of fatty acids are considered essential, the omega-3 and omega-6 fatty acids. Essential fatty acids are necessary for humans but cannot be synthesized by the body and must therefore be obtained from food. Omega-3 and omega-6 are used in some cellular signaling pathways and are involved in mediating inflammation, protein synthesis, and metabolic pathways in the human body.
Docosapentaenoic acid (DPA) designates any straight open chain polyunsaturated fatty acid (PUFA) which contains 22 carbons and 5 double bonds. DPA is primarily used to designate two isomers, all-cis-4,7,10,13,16-docosapentaenoic acid and all-cis-7,10,13,16,19-docosapentaenoic acid. They are also commonly termed n-6 DPA and n-3 DPA, respectively; these designations describe the position of the double bond being 6 or 3 carbons closest to the (omega) carbon at the methyl end of the molecule and is based on the biologically important difference that n-6 and n-3 PUFA are separate PUFA classes, i.e. the omega-6 fatty acids and omega-3 fatty acids, respectively. Mammals, including humans, can not interconvert these two classes and therefore must obtain dietary essential PUFA fatty acids from both classes in order to maintain normal health.

Linoleoyl-CoA desaturase (also Delta 6 desaturase, EC 1.14.19.3) is an enzyme that converts between types of fatty acids, which are essential nutrients in the human body. The enzyme mainly catalyzes the chemical reaction

Cytochrome P450 4F8 is a protein that in humans is encoded by the CYP4F8 gene.

Omega-3-acid ethyl esters are a mixture of ethyl eicosapentaenoic acid and ethyl docosahexaenoic acid, which are ethyl esters of the omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) found in fish oil. Together with dietary changes, they are used to treat high blood triglycerides which may reduce the risk of pancreatitis. They are generally less preferred than statins, and use is not recommended by NHS Scotland as the evidence does not support a decreased risk of heart disease. Omega-3-acid ethyl esters are taken by mouth.
Omega-3 carboxylic acids (Epanova) is a formerly marketed yet still not an Food And Drug Administration (FDA) approved prescription medication–since taken off market by the manufacturer–used alongside a low fat and low cholesterol diet that lowers high triglyceride (fat) levels in adults with very high levels. This was the third class of fish oil-based drug, after omega-3 acid ethyl esters and ethyl eicosapentaenoic acid (Vascepa), to be approved for use as a drug. The first approval by US Food and Drug Administration was granted 05 May 2014. These fish oil drugs are similar to fish oil dietary supplements, but the ingredients are better controlled and have been tested in clinical trials. Specifically, Epanova contained at least 850 mg omega-3-acid ethyl esters per 1 g capsule.
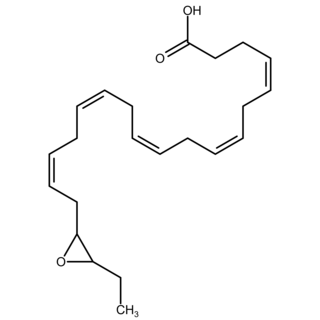
Epoxide docosapentaenoic acids are metabolites of the 22-carbon straight-chain omega-3 fatty acid, docosahexaenoic acid (DHA). Cell types that express certain cytochrome P450 (CYP) epoxygenases metabolize polyunsaturated fatty acids (PUFAs) by converting one of their double bonds to an epoxide. In the best known of these metabolic pathways, cellular CYP epoxygenases metabolize the 20-carbon straight-chain omega-6 fatty acid, arachidonic acid, to epoxyeicosatrienoic acids (EETs); another CYP epoxygenase pathway metabolizes the 20-carbon omega-3 fatty acid, eicosapentaenoic acid (EPA), to epoxyeicosatetraenoic acids (EEQs). CYP epoxygenases similarly convert various other PUFAs to epoxides. These epoxide metabolites have a variety of activities. However, essentially all of them are rapidly converted to their corresponding, but in general far less active, vicinal dihydroxy fatty acids by ubiquitous cellular soluble epoxide hydrolase. Consequently, these epoxides, including EDPs, operate as short-lived signaling agents that regulate the function of their parent or nearby cells. The particular feature of EDPs distinguishing them from EETs is that they derive from omega-3 fatty acids and are suggested to be responsible for some of the beneficial effects attributed to omega-3 fatty acids and omega-3-rich foods such as fish oil.

Epoxyeicosatetraenoic acids are a set of biologically active epoxides that various cell types make by metabolizing the omega 3 fatty acid, eicosapentaenoic acid (EPA), with certain cytochrome P450 epoxygenases. These epoxygenases can metabolize EPA to as many as 10 epoxides that differ in the site and/or stereoisomer of the epoxide formed; however, the formed EEQs, while differing in potency, often have similar bioactivities and are commonly considered together.
In general, cognitive support diets are formulated to include nutrients that have a known role in brain development, function and/or maintenance, with the goal of improving and preserving mental processes such as attentiveness, short-term and long-term memory, learning, and problem solving. Currently, there is very little conclusive research available regarding cat cognition as standardized tests for evaluating cognitive ability are less established and less reliable than cognitive testing apparatus used in other mammalian species, like dogs. Much of what is known about feline cognition has been inferred from a combination of owner-reported behaviour, brain necropsies, and comparative cognitive neurology of related animal models. Cognition claims appear primarily on kitten diets which include elevated levels of nutrients associated with optimal brain development, although there are now diets available for senior cats that include nutrients to help slow the progression of age-related changes and prevent cognitive decline. Cognition diets for cats contain a greater portion of omega-3 fatty acids, especially docosahexaenoic acid (DHA) as well as eicosapentaenoic acid (EPA), and usually feature a variety of antioxidants and other supporting nutrients thought to have positive effects on cognition.

Seaweed oil, also called algae oil or algal oil, is used for making food, with the purified product almost colorless and odorless. It is also under development as a possible alternative fuel and manufacturing agent.