
In nutrition, biology, and chemistry, fat usually means any ester of fatty acids, or a mixture of such compounds, most commonly those that occur in living beings or in food.
Essential fatty acids, or EFAs, are fatty acids that humans and other animals must ingest because the body requires them for good health, but cannot synthesize them.

α-Linolenic acid, also known as alpha-Linolenic acid (ALA), is an n−3, or omega-3, essential fatty acid. ALA is found in many seeds and oils, including flaxseed, walnuts, chia, hemp, and many common vegetable oils.
Linoleic acid (LA) is an organic compound with the formula HOOC(CH2)7CH=CHCH2CH=CH(CH2)4CH3. Both alkene groups are cis. It is a fatty acid sometimes denoted 18:2 (n-6) or 18:2 cis-9,12. A linoleate is a salt or ester of this acid.

Conjugated linoleic acids (CLA) are a family of isomers of linoleic acid. In principle, 28 isomers are possible. CLA is found mostly in the meat and dairy products derived from ruminants. The two C=C double bonds are conjugated. CLAs can be either cis-fats or trans-fats.

In biochemistry and nutrition, a polyunsaturated fat is a fat that contains a polyunsaturated fatty acid, which is a subclass of fatty acid characterized by a backbone with two or more carbon–carbon double bonds. Some polyunsaturated fatty acids are essentials. Polyunsaturated fatty acids are precursors to and are derived from polyunsaturated fats, which include drying oils.
Fatty acid desaturases are a family of enzymes that convert saturated fatty acids into unsaturated fatty acids and polyunsaturated fatty acids. For the common fatty acids of the C18 variety, desaturases convert stearic acid into oleic acid. Other desaturases convert oleic acid into linolenic acid, which is the precursor to alpha-linolenic acid, gamma-linolenic acid, and eicosatrienoic acid.
Vaccenic acid is a naturally occurring trans fatty acid and an omega-7 fatty acid. It is the predominant kind of trans-fatty acid found in human milk, in the fat of ruminants, and in dairy products such as milk, butter, and yogurt. Trans fat in human milk may depend on trans fat content in food.
Calendic acid is an unsaturated fatty acid, named for the pot marigold, from which it is obtained. It is chemically similar to the conjugated linoleic acids; laboratory work suggests it may have similar in vitro bioactivities.

Rumenic acid, also known as bovinic acid, is a conjugated linoleic acid (CLA) found in the fat of ruminants and in dairy products. It is an omega-7 trans fatty acid. Its lipid shorthand name is cis-9, trans-11 18:2 acid. The name was proposed by Kramer et al. in 1998. It can be considered as the principal dietary form, accounting for as much as 85-90% of the total CLA content in dairy products.

α-Eleostearic acid or (9Z,11E,13E)-octadeca-9,11,13-trienoic acid, is an organic compound, a conjugated fatty acid and one of the isomers of octadecatrienoic acid. It is often called simply eleostearic acid although there is also a β-eleostearic acid. Its high degree of unsaturation gives tung oil its properties as a drying oil.

Conjugated fatty acids is jargon for polyunsaturated fatty acids containing at least one pair of conjugated double bonds. An example of a conjugated fatty acid is the rumenic acid, found in the meat and milk of ruminants. Most unsaturated fatty acids that are doubly unsaturated do not feature conjugation, e.g., linoleic acid and linoelaidic acid.

Mead acid is an omega-9 fatty acid, first characterized by James F. Mead. As with some other omega-9 polyunsaturated fatty acids, animals can make Mead acid de novo. Its elevated presence in the blood is an indication of essential fatty acid deficiency. Mead acid is found in large quantities in cartilage.

Linoleoyl-CoA desaturase (also Delta 6 desaturase, EC 1.14.19.3) is an enzyme that converts between types of fatty acids, which are essential nutrients in the human body. The enzyme mainly catalyzes the chemical reaction

α-Parinaric acid is a conjugated polyunsaturated fatty acid. Discovered by Tsujimoto and Koyanagi in 1933, it contains 18 carbon atoms and 4 conjugated double bonds. The repeating single bond-double bond structure of α-parinaric acid distinguishes it structurally and chemically from the usual "methylene-interrupted" arrangement of polyunsaturated fatty acids that have double-bonds and single bonds separated by a methylene unit (−CH2−). Because of the fluorescent properties conferred by the alternating double bonds, α-parinaric acid is commonly used as a molecular probe in the study of biomembranes.

An octadecatrienoic acid is a chemical compound with formula C
18H
30O
2, a polyunsaturated fatty acid whose molecule has an 18-carbon unbranched backbone with three double bonds.
Only two essential fatty acids are known to be essential for humans: alpha-linolenic acid and linoleic acid. The biological effects of the ω-3 and ω-6 fatty acids are mediated by their mutual interactions. Closely related, these fatty acids act as competing substrates for the same enzymes. The biological effects of the ω-3 and ω-6 fatty acids are largely mediated by essential fatty acid interactions. The proportion of omega-3 to omega-6 fatty acids in a diet may have metabolic consequences. Unlike omega-3 fatty acids and omega-6 fatty acids, omega-9 fatty acids are not classed as essential fatty acids because they can be created by the human body from monounsaturated and saturated fatty acids, and are therefore not essential in the diet.
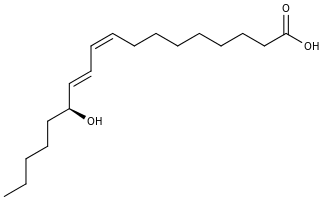
13-Hydroxyoctadecadienoic acid (13-HODE) is the commonly used term for 13(S)-hydroxy-9Z,11E-octadecadienoic acid (13(S)-HODE). The production of 13(S)-HODE is often accompanied by the production of its stereoisomer, 13(R)-hydroxy-9Z,11E-octadecadienoic acid (13(R)-HODE). The adjacent figure gives the structure for the (S) stereoisomer of 13-HODE. Two other naturally occurring 13-HODEs that may accompany the production of 13(S)-HODE are its cis-trans (i.e., 9E,11E) isomers viz., 13(S)-hydroxy-9E,11E-octadecadienoic acid (13(S)-EE-HODE) and 13(R)-hydroxy-9E,11E-octadecadienoic acid (13(R)-EE-HODE). Studies credit 13(S)-HODE with a range of clinically relevant bioactivities; recent studies have assigned activities to 13(R)-HODE that differ from those of 13(S)-HODE; and other studies have proposed that one or more of these HODEs mediate physiological and pathological responses, are markers of various human diseases, and/or contribute to the progression of certain diseases in humans. Since, however, many studies on the identification, quantification, and actions of 13(S)-HODE in cells and tissues have employed methods that did not distinguish between these isomers, 13-HODE is used here when the actual isomer studied is unclear.

Coronaric acid (leukotoxin or leukotoxin A) is a mono-unsaturated, epoxide derivative of the di-saturated fatty acid, linoleic acid (i.e. 9(Z),12(Z) octadecadienoic acid). It is a mixture of the two optically active isomers of 12(Z) 9,10-epoxy-octadecenoic acid. This mixture is also termed 9,10-epoxy-12Z-octadecenoic acid or 9(10)-EpOME and when formed by or studied in mammalians, leukotoxin.

Reinforced lipids are lipid molecules in which some of the fatty acids contain deuterium instead of hydrogen. They can be used for the protection of living cells by slowing the chain reaction due to isotope effect on lipid peroxidation. The lipid bilayer of the cell and organelle membranes contain polyunsaturated fatty acids (PUFA) are key components of cell and organelle membranes. Any process that either increases oxidation of PUFAs or hinders their ability to be replaced can lead to serious disease. Correspondingly, use of reinforced lipids that stop the chain reaction of lipid peroxidation has preventive and therapeutic potential.












