
In chemistry, an ester is a compound derived from an acid in which the hydrogen atom (H) of at least one acidic hydroxyl group of that acid is replaced by an organyl group. Chalcogen analogues are included. By some authors, organyl derivatives of acidic hydrogen of other acids are called esters as well, but those compounds do not belong to the class "esters" proper, according to the IUPAC. An example of an ester formation is the substitution reaction of a carboxylic acid and an alcohol (R'OH), forming an ester, where R and R′ denote organyl groups, or H in the case of R. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils. Esters can be formed from oxoacids, but also from acids that do not contain oxygen.

Valerian is a perennial flowering plant native to Europe and Asia. In the summer when the mature plant may have a height of 1.5 metres, it bears sweetly scented pink or white flowers that attract many fly species, especially hoverflies of the genus Eristalis. It is consumed as food by the larvae of some Lepidoptera species, including the grey pug.

Isobutyric acid, also known as 2-methylpropanoic acid or isobutanoic acid, is a carboxylic acid with structural formula (CH3)2CHCOOH. It is an isomer of n-butyric acid. It is classified as a short-chain fatty acid. Deprotonation or esterification gives derivatives called isobutyrates.

Levulinic acid, or 4-oxopentanoic acid, is an organic compound with the formula CH3C(O)CH2CH2CO2H. It is classified as a keto acid. This white crystalline solid is soluble in water and polar organic solvents. It is derived from degradation of cellulose and is a potential precursor to biofuels, such as ethyl levulinate.
Enanthic acid, also called heptanoic acid, is an organic compound composed of a seven-carbon chain terminating in a carboxylic acid functional group. It is a colorless oily liquid with an unpleasant, rancid odor. It contributes to the odor of some rancid oils. It is slightly soluble in water, but very soluble in ethanol and ether. Salts and esters of enanthic acid are called enanthates or heptanoates.

3-Methylbutanoic acid, also known as β-methylbutyric acid or more commonly isovaleric acid, is a branched-chain alkyl carboxylic acid with the chemical formula (CH3)2CHCH2CO2H. It is classified as a short-chain fatty acid. Like other low-molecular-weight carboxylic acids, it has an unpleasant odor. The compound occurs naturally and can be found in many foods, such as cheese, soy milk, and apple juice.

Ethyl pentanoate, also commonly known as ethyl valerate, is an organic compound used in flavors. It is an ester with the molecular formula C7H14O2. This colorless liquid is poorly soluble in water but miscible with organic solvents.
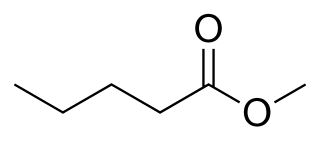
Methyl pentanoate, commonly known as methyl valerate, is the methyl ester of pentanoic acid with a fruity odor.

Ethyl acrylate is an organic compound with the formula CH2CHCO2CH2CH3. It is the ethyl ester of acrylic acid. It is a colourless liquid with a characteristic acrid odor. It is mainly produced for paints, textiles, and non-woven fibers. It is also a reagent in the synthesis of various pharmaceutical intermediates.

Ethyl propionate is an organic compound with formula C2H5O2CCH2CH3. It is the ethyl ester of propionic acid. It is a colorless volatile liquid with a pineapple-like odor. Some fruits such as kiwis and strawberries contain ethyl propionate in small amounts.

tert-Butylthiol, also known as 2-methylpropane-2-thiol, 2-methyl-2-propanethiol, tert-butyl mercaptan (TBM), and t-BuSH, is an organosulfur compound with the formula (CH3)3CSH. This thiol is used as an odorant for natural gas, which is otherwise odorless. It may also have been used as a flavoring agent.

4-Methylcyclohexanemethanol (MCHM, systematic name 4-methylcyclohexylmethanol) is an organic compound with the formula CH3C6H10CH2OH. Classified as a saturated higher alicyclic primary alcohol. Both cis and trans isomers exist, depending on the relative positions of the methyl (CH3) and hydroxymethyl (CH2OH) groups on the cyclohexane ring. Commercial samples of MCHM consists of a mixture of these isomers as well as other components that vary with the supplier.

A GABA analogue is a compound which is an analogue or derivative of the neurotransmitter gamma-Aminobutyric acid (GABA).
Ethyl octanoate, also known as ethyl caprylate, is a fatty acid ester formed from caprylic acid and ethanol. A colorless liquid at room temperature, it has the semi-developed formula of CH3(CH2)6COOCH2CH3, and is used in food industries as a flavoring and in the perfume industry as a scent additive. It is present in many fruits and alcoholic beverages, and has a strong odor of fruit and flowers. It is used in the creation of synthetic fruity scents.

Ethyl isovalerate is an organic compound that is the ester formed from ethyl alcohol and isovaleric acid. It has a fruity odor and flavor and is used in perfumery and as a food additive.
2-Methylbutanoic acid, also known as 2-methylbutyric acid is a branched-chain alkyl carboxylic acid with the chemical formula CH3CH2CH(CH3)CO2H, classified as a short-chain fatty acid. It exists in two enantiomeric forms, (R)- and (S)-2-methylbutanoic acid. (R)-2-methylbutanoic acid occurs naturally in cocoa beans and (S)-2-methylbutanoic occurs in many fruits such as apples and apricots, as well as in the scent of the orchid Luisia curtisii.
Methyl hexanoate is the fatty acid methyl ester of hexanoic acid, a colourless liquid organic compound with the chemical formula CH3−(CH2)4−COO−CH3. It is found naturally in many foods and has a role as a plant metabolite. It can also be found in the cytoplasm of cells.
Pentenoic acid is any of five mono-carboxylic acids whose molecule has an unbranched chain of five carbons connected by three single bonds and one double bond. That is, any compound with one of the formulas HO(O=)C−CH=CH−CH2−CH3 (2-pentenoic), HO(O=)C−CH2−CH=CH−CH3 (3-pentenoic), or HO(O=)C−CH2−CH2−CH=CH2 (4-pentenoic). In the IUPAC-recommended nomenclature, these acids are called pent-2-enoic, pent-3-enoic, and pent-4-enoic, respectively. All these compounds have the empirical formula C
5H
8O
2.
















