Essential fatty acids, or EFAs, are fatty acids that humans and other animals must ingest because the body requires them for good health, but cannot synthesize them.

Arachidonic acid is a polyunsaturated omega-6 fatty acid 20:4(ω-6), or 20:4(5,8,11,14). It is structurally related to the saturated arachidic acid found in cupuaçu butter. Its name derives from the ancient Greek neologism arachis (peanut), but peanut oil does not contain any arachidonic acid.
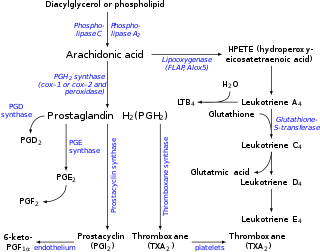
Eicosanoids are signaling molecules made by the enzymatic or non-enzymatic oxidation of arachidonic acid or other polyunsaturated fatty acids (PUFAs) that are, similar to arachidonic acid, around 20 carbon units in length. Eicosanoids are a sub-category of oxylipins, i.e. oxidized fatty acids of diverse carbon units in length, and are distinguished from other oxylipins by their overwhelming importance as cell signaling molecules. Eicosanoids function in diverse physiological systems and pathological processes such as: mounting or inhibiting inflammation, allergy, fever and other immune responses; regulating the abortion of pregnancy and normal childbirth; contributing to the perception of pain; regulating cell growth; controlling blood pressure; and modulating the regional flow of blood to tissues. In performing these roles, eicosanoids most often act as autocrine signaling agents to impact their cells of origin or as paracrine signaling agents to impact cells in the proximity of their cells of origin. Eicosanoids may also act as endocrine agents to control the function of distant cells.

Lipoxygenases (LOX) are a family of (non-heme) iron-containing enzymes, more specifically oxidative enzymes, most of which catalyze the dioxygenation of polyunsaturated fatty acids in lipids containing a cis,cis-1,4-pentadiene into cell signaling agents that serve diverse roles as autocrine signals that regulate the function of their parent cells, paracrine signals that regulate the function of nearby cells, and endocrine signals that regulate the function of distant cells.
Fatty acid desaturases are a family of enzymes that convert saturated fatty acids into unsaturated fatty acids and polyunsaturated fatty acids. For the common fatty acids of the C18 variety, desaturases convert stearic acid into oleic acid. Other desaturases convert oleic acid into linolenic acid, which is the precursor to alpha-linolenic acid, gamma-linolenic acid, and eicosatrienoic acid.
There are many fatty acids found in nature. Two types of fatty acids considered essential for human health are the omega-3 and omega-6 types. These two essential fatty acids are necessary for some cellular signalling pathways and are involved in mediating inflammation, protein synthesis, and metabolic pathways in the human body.
Omega oxidation (ω-oxidation) is a process of fatty acid metabolism in some species of animals. It is an alternative pathway to beta oxidation that, instead of involving the β carbon, involves the oxidation of the ω carbon. The process is normally a minor catabolic pathway for medium-chain fatty acids, but becomes more important when β oxidation is defective.
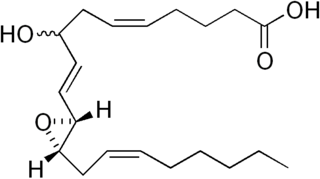
Hepoxilins (Hx) are a set of epoxyalcohol metabolites of polyunsaturated fatty acids (PUFA), i.e. they possess both an epoxide and an alcohol residue. HxA3, HxB3, and their non-enzymatically formed isomers are nonclassic eicosanoid derived from acid the (PUFA), arachidonic acid. A second group of less well studied hepoxilins, HxA4, HxB4, and their non-enzymatically formed isomers are nonclassical eicosanoids derived from the PUFA, eicosapentaenoic acid. Recently, 14,15-HxA3 and 14,15-HxB3 have been defined as arachidonic acid derivatives that are produced by a different metabolic pathway than HxA3, HxB3, HxA4, or HxB4 and differ from the aforementioned hepoxilins in the positions of their hydroxyl and epoxide residues. Finally, hepoxilin-like products of two other PUFAs, docosahexaenoic acid and linoleic acid, have been described. All of these epoxyalcohol metabolites are at least somewhat unstable and are readily enzymatically or non-enzymatically to their corresponding trihydroxy counterparts, the trioxilins (TrX). HxA3 and HxB3, in particular, are being rapidly metabolized to TrXA3, TrXB3, and TrXC3. Hepoxilins have various biological activities in animal models and/or cultured mammalian tissues and cells. The TrX metabolites of HxA3 and HxB3 have less or no activity in most of the systems studied but in some systems retain the activity of their precursor hepoxilins. Based on these studies, it has been proposed that the hepoxilins and trioxilins function in human physiology and pathology by, for example, promoting inflammation responses and dilating arteries to regulate regional blood flow and blood pressure.
Arachidonate 5-lipoxygenase, also known as ALOX5, 5-lipoxygenase, 5-LOX, or 5-LO, is a non-heme iron-containing enzyme that in humans is encoded by the ALOX5 gene. Arachidonate 5-lipoxygenase is a member of the lipoxygenase family of enzymes. It transforms essential fatty acids (EFA) substrates into leukotrienes as well as a wide range of other biologically active products. ALOX5 is a current target for pharmaceutical intervention in a number of diseases.

ALOX15 is, like other lipoxygenases, a seminal enzyme in the metabolism of polyunsaturated fatty acids to a wide range of physiologically and pathologically important products. ▼ Gene Function

ALOX12, also known as arachidonate 12-lipoxygenase, 12-lipoxygenase, 12S-Lipoxygenase, 12-LOX, and 12S-LOX is a lipoxygenase-type enzyme that in humans is encoded by the ALOX12 gene which is located along with other lipoyxgenases on chromosome 17p13.3. ALOX12 is 75 kilodalton protein composed of 663 amino acids.

Oxoeicosanoid receptor 1 (OXER1) also known as G-protein coupled receptor 170 (GPR170) is a protein that in humans is encoded by the OXER1 gene located on human chromosome 2p21; it is the principal receptor for the 5-Hydroxyicosatetraenoic acid family of carboxy fatty acid metabolites derived from arachidonic acid. The receptor has also been termed hGPCR48, HGPCR48, and R527 but OXER1 is now its preferred designation. OXER1 is a G protein-coupled receptor (GPCR) that is structurally related to the hydroxy-carboxylic acid (HCA) family of G protein-coupled receptors whose three members are HCA1 (GPR81), HCA2, and HCA3 ; OXER1 has 30.3%, 30.7%, and 30.7% amino acid sequence identity with these GPCRs, respectively. It is also related to the recently defined receptor, GPR31, for the hydroxyl-carboxy fatty acid 12-HETE.

5-Hydroxyeicosatetraenoic acid (5-HETE, 5(S)-HETE, or 5S-HETE) is an eicosanoid, i.e. a metabolite of arachidonic acid. It is produced by diverse cell types in humans and other animal species. These cells may then metabolize the formed 5(S)-HETE to 5-oxo-eicosatetraenoic acid (5-oxo-ETE), 5(S),15(S)-dihydroxyeicosatetraenoic acid (5(S),15(S)-diHETE), or 5-oxo-15-hydroxyeicosatetraenoic acid (5-oxo-15(S)-HETE).
Epoxygenases are a set of membrane-bound, heme-containing cytochrome P450 enzymes that metabolize polyunsaturated fatty acids to epoxide products that have a range of biological activities. The most thoroughly studied substrate of the CYP epoxylgenases is arachidonic acid. This polyunsaturated fatty acid is metabolized by cyclooxygenases to various prostaglandin, thromboxane, and prostacyclin metabolites in what has been termed the first pathway of eicosanoid production; it is also metabolized by various lipoxygenases to hydroxyeicosatetraenoic acids and leukotrienes in what has been termed the second pathway of eicosanoid production. The metabolism of arachidonic acid to epoxyeicosatrienoic acids by the CYP epoxygenases has been termed the third pathway of eicosanoid metabolism. Like the first two pathways of eicosanoid production, this third pathway acts as a signaling pathway wherein a set of enzymes metabolize arachidonic acid to a set of products that act as secondary signals to work in activating their parent or nearby cells and thereby orchestrate functional responses. However, none of these three pathways is limited to metabolizing arachidonic acid to eicosanoids. Rather, they also metabolize other polyunsaturated fatty acids to products that are structurally analogous to the eicosanoids but often have different bioactivity profiles. This is particularly true for the CYP epoxygenases which in general act on a broader range of polyunsaturated fatty acids to form a broader range of metabolites than the first and second pathways of eicosanoid production. Furthermore, the latter pathways form metabolites many of which act on cells by binding with and thereby activating specific and well-characterized receptor proteins; no such receptors have been fully characterized for the epoxide metabolites. Finally, there are relatively few metabolite-forming lipoxygenases and cyclooxygenases in the first and second pathways and these oxygenase enzymes share similarity between humans and other mammalian animal models. The third pathway consists of a large number of metabolite-forming CYP epoxygenases and the human epoxygenases have important differences from those of animal models. Partly because of these differences, it has been difficult to define clear roles for the epoxygenase-epoxide pathways in human physiology and pathology.
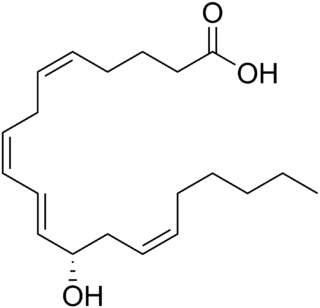
12-Hydroxyeicosatetraenoic acid (12-HETE) is a derivative of the 20 carbon polyunsaturated fatty acid, arachidonic acid, containing a hydroxyl residue at carbon 12 and a 5Z,8Z,10E,14Z Cis–trans isomerism configuration (Z=cis, E=trans) in its four double bonds. It was first found as a product of arachidonic acid metabolism made by human and bovine platelets through their 12S-lipoxygenase (i.e. ALOX12) enzyme(s). However, the term 12-HETE is ambiguous in that it has been used to indicate not only the initially detected "S" stereoisomer, 12S-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic acid (12(S)-HETE or 12S-HETE), made by platelets, but also the later detected "R" stereoisomer, 12(R)-hydroxy-5Z,8Z,10E,14Z-eicosatetraenoic acid (also termed 12(R)-HETE or 12R-HETE) made by other tissues through their 12R-lipoxygenase enzyme, ALOX12B. The two isomers, either directly or after being further metabolized, have been suggested to be involved in a variety of human physiological and pathological reactions. Unlike hormones which are secreted by cells, travel in the circulation to alter the behavior of distant cells, and thereby act as Endocrine signalling agents, these arachidonic acid metabolites act locally as Autocrine signalling and/or Paracrine signaling agents to regulate the behavior of their cells of origin or of nearby cells, respectively. In these roles, they may amplify or dampen, expand or contract cellular and tissue responses to disturbances.
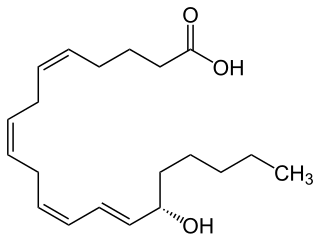
15-Hydroxyeicosatetraenoic acid (also termed 15-HETE, 15(S)-HETE, and 15S-HETE) is an eicosanoid, i.e. a metabolite of arachidonic acid. Various cell types metabolize arachidonic acid to 15(S)-hydroperoxyeicosatetraenoic acid (15(S)-HpETE). This initial hydroperoxide product is extremely short-lived in cells: if not otherwise metabolized, it is rapidly reduced to 15(S)-HETE. Both of these metabolites, depending on the cell type which forms them, can be further metabolized to 15-oxo-eicosatetraenoic acid (15-oxo-ETE), 5(S),15(S)-dihydroxy-eicosatetraenoic acid (5(S),15(S)-diHETE), 5-oxo-15(S)-hydroxyeicosatetraenoic acid (5-oxo-15(S)-HETE), a subset of specialized pro-resolving mediators viz., the lipoxins, a class of pro-inflammatory mediators, the eoxins, and other products that have less well-defined activities and functions. Thus, 15(S)-HETE and 15(S)-HpETE, in addition to having intrinsic biological activities, are key precursors to numerous biologically active derivatives.
Eoxins are proposed to be a family of proinflammatory eicosanoids. They are produced by human eosinophils, mast cells, the L1236 Reed–Sternberg cell line derived from Hodgkin's lymphoma, and certain other tissues. These cells produce the eoxins by initially metabolizing arachidonic acid, an omega-6 (ω-6) fatty acid, via any enzyme possessing 15-lipoxygenase activity. The product of this initial metabolic step, 15(S)-hydroperoxyeicosatetraenoic acid, is then converted to a series of eoxins by the same enzymes that metabolize the 5-lipoxygenase product of arachidonic acid metabolism, i.e. 5-Hydroperoxy-eicosatetraenoic acid to a series of leukotrienes. That is, the eoxins are 14,15-disubstituted analogs of the 5,6-disubstituted leukotrienes.
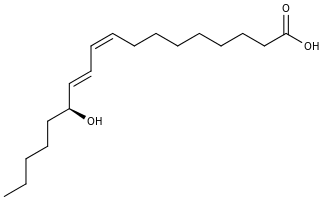
13-Hydroxyoctadecadienoic acid (13-HODE) is the commonly used term for 13(S)-hydroxy-9Z,11E-octadecadienoic acid (13(S)-HODE). The production of 13(S)-HODE is often accompanied by the production of its stereoisomer, 13(R)-hydroxy-9Z,11E-octadecadienoic acid (13(R)-HODE). The adjacent figure gives the structure for the (S) stereoisomer of 13-HODE. Two other naturally occurring 13-HODEs that may accompany the production of 13(S)-HODE are its cis-trans (i.e., 9E,11E) isomers viz., 13(S)-hydroxy-9E,11E-octadecadienoic acid (13(S)-EE-HODE) and 13(R)-hydroxy-9E,11E-octadecadienoic acid (13(R)-EE-HODE). Studies credit 13(S)-HODE with a range of clinically relevant bioactivities; recent studies have assigned activities to 13(R)-HODE that differ from those of 13(S)-HODE; and other studies have proposed that one or more of these HODEs mediate physiological and pathological responses, are markers of various human diseases, and/or contribute to the progression of certain diseases in humans. Since, however, many studies on the identification, quantification, and actions of 13(S)-HODE in cells and tissues have employed methods that did not distinguish between these isomers, 13-HODE is used here when the actual isomer studied is unclear.

5-Oxo-eicosatetraenoic acid is a nonclassic eicosanoid metabolite of arachidonic acid and the most potent naturally occurring member of the 5-HETE family of cell signaling agents. Like other cell signaling agents, 5-oxo-ETE is made by a cell and then feeds back to stimulate its parent cell and/or exits this cell to stimulate nearby cells. 5-Oxo-ETE can stimulate various cell types particularly human leukocytes but possesses its highest potency and power in stimulating the human eosinophil type of leukocyte. It is therefore suggested to be formed during and to be an important contributor to the formation and progression of eosinophil-based allergic reactions; it is also suggested that 5-oxo-ETE contributes to the development of inflammation, cancer cell growth, and other pathological and physiological events.

Epoxyeicosatetraenoic acids are a set of biologically active epoxides that various cell types make by metabolizing the omega 3 fatty acid, eicosapentaenoic acid (EPA), with certain cytochrome P450 epoxygenases. These epoxygenases can metabolize EPA to as many as 10 epoxides that differ in the site and/or stereoisomer of the epoxide formed; however, the formed EEQs, while differing in potency, often have similar bioactivities and are commonly considered together.














