
An ester is a chemical compound derived from an acid in which at least one –OH hydroxyl group is replaced by an –O– alkyl (alkoxy) group, as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils.
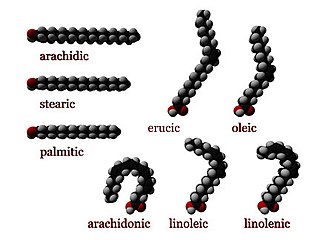
In chemistry, particularly in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated or unsaturated. Most naturally occurring fatty acids have an unbranched chain of an even number of carbon atoms, from 4 to 28. Fatty acids are a major component of the lipids in some species such as microalgae but in some other organisms are not found in their standalone form, but instead exist as three main classes of esters: triglycerides, phospholipids, and cholesteryl esters. In any of these forms, fatty acids are both important dietary sources of fuel for animals and important structural components for cells.

A triglyceride is an ester derived from glycerol and three fatty acids. Triglycerides are the main constituents of body fat in humans and other vertebrates, as well as vegetable fat. They are also present in the blood to enable the bidirectional transference of adipose fat and blood glucose from the liver, and are a major component of human skin oils.

α-Linolenic acid (ALA),, is an n−3, or omega-3, essential fatty acid. ALA is found in many seeds and oils, including flaxseed, walnuts, chia, hemp, and many common vegetable oils.
Erucic acid is a monounsaturated omega-9 fatty acid, denoted 22:1ω9. It has the chemical formula CH3(CH2)7CH=CH(CH2)11COOH. It is prevalent in wallflower seed and other plants in the family Brassicaceae, with a reported content of 20 to 54% in high erucic acid rapeseed oil and 42% in mustard oil. Erucic acid is also known as cis-13-docosenoic acid and the trans isomer is known as brassidic acid.

Oleic acid is a fatty acid that occurs naturally in various animal and vegetable fats and oils. It is an odorless, colorless oil, although commercial samples may be yellowish. In chemical terms, oleic acid is classified as a monounsaturated omega-9 fatty acid, abbreviated with a lipid number of 18:1 cis-9. It has the formula CH3(CH2)7CH=CH(CH2)7COOH. The name derives from the Latin word oleum, which means oil. It is the most common fatty acid in nature. The salts and esters of oleic acid are called oleates.
Biodiesel production is the process of producing the biofuel, biodiesel, through the chemical reactions of transesterification and esterification. This involves vegetable or animal fats and oils being reacted with short-chain alcohols. The alcohols used should be of low molecular weight. Ethanol is the most used because of its low cost, however, greater conversions into biodiesel can be reached using methanol. Although the transesterification reaction can be catalyzed by either acids or bases, the base-catalyzed reaction is more common. This path has lower reaction times and catalyst cost than those acid catalysis. However, alkaline catalysis has the disadvantage of high sensitivity to both water and free fatty acids present in the oils.

Garryaceae is a small family of plants known commonly as the silktassels. It contains two genera:

Oleyl alcohol, or cis-9-octadecen-1-ol, is an unsaturated fatty alcohol with the molecular formula C18H36O or the condensed structural formula CH3(CH2)7−CH=CH−(CH2)8OH. It is a colorless oil, mainly used in cosmetics.
Fatty alcohols (or long-chain alcohols) are usually high-molecular-weight, straight-chain primary alcohols, but can also range from as few as 4–6 carbons to as many as 22–26, derived from natural fats and oils. The precise chain length varies with the source. Some commercially important fatty alcohols are lauryl, stearyl, and oleyl alcohols. They are colourless oily liquids (for smaller carbon numbers) or waxy solids, although impure samples may appear yellow. Fatty alcohols usually have an even number of carbon atoms and a single alcohol group (–OH) attached to the terminal carbon. Some are unsaturated and some are branched. They are widely used in industry. As with fatty acids, they are often referred to generically by the number of carbon atoms in the molecule, such as "a C12 alcohol", that is an alcohol having 12 carbons, for example dodecanol.

The Simaroubaceae are a small, mostly tropical, family in the order Sapindales. In recent decades, it has been subject to much taxonomic debate, with several small families being split off. A molecular phylogeny of the family was published in 2007, greatly clarifying relationships within the family. Together with chemical characteristics such as the occurrence of petroselinic acid in Picrasma in contrast to other members of the family such as Ailanthus this indicates the existence of a subgroup in the family with Picrasma, Holacantha, and Castela.

Sunflower oil is the non-volatile oil pressed from the seeds of sunflower. Sunflower oil is commonly used in food as a frying oil, and in cosmetic formulations as an emollient.

Monoglycerides are a class of glycerides which are composed of a molecule of glycerol linked to a fatty acid via an ester bond. As glycerol contains both primary and secondary alcohol groups two different types of monoglycerides may be formed; 1-monoacylglycerols where the fatty acid is attached to a primary alcohol, or a 2-monoacylglycerols where the fatty acid is attached to the secondary alcohol.
Elaidic acid is a chemical compound with the formula C
18H
34O
2, specifically the fatty acid with structural formula HO(O=)C–(CH2–)7CH=CH–(CH2–)8H, with the double bond (between carbon atoms 9 and 10) in trans configuration. It is a colorless oily solid. Its salts and esters are called elaidates.
Stillingia oil is an oil extracted from the seeds of plants of the Triadica genus such as Triadica sebifera and Triadica cochinchinensis. It is a drying oil used in paints and varnishes, and it is believed to be toxic in China. It must be distinguished from stillingia tallow, a fatty substance that surround the seeds in the fruit and must be removed before extracting the oil.
Oleochemistry is the study of vegetable oils and animal oils and fats, and oleochemicals derived from these fats and oils. The resulting product can be called oleochemicals (from Latin: oleum "olive oil"). The major product of this industry is soap, approximately 8.9×106 tons of which were produced in 1990. Other major oleochemicals include fatty acids, fatty acid methyl esters, fatty alcohols and fatty amines. Glycerol is a side product of all of these processes. Intermediate chemical substances produced from these basic oleochemical substances include alcohol ethoxylates, alcohol sulfates, alcohol ether sulfates, quaternary ammonium salts, monoacylglycerols (MAG), diacylglycerols (DAG), structured triacylglycerols (TAG), sugar esters, and other oleochemical products.
Tariric acid is an acetylenic fatty acid that can be found in the tallow-wood tree, Ximenia americana.

Kusum oil is a type of oil extracted from the seed of the Kusum tree. The plant, which is also commonly known as Ceylon oak, lac tree, or Macassar oiltree, belongs to the family Sapindaceae. The schleichera family is named after J. C. Schleicher, a Swiss botanist, and the species name means "oily" or "rich in oil." The tree is native to South Asia, but is also found in some parts of Southeast Asia.

Cooking oil is plant, animal, or synthetic fat used in frying, baking, and other types of cooking. It is also used in food preparation and flavoring not involving heat, such as salad dressings and bread dips, and may be called edible oil.
Decadienoic acid is any mono-carboxylic acid with an unbranched chain of ten carbon atoms, connected by seven single bonds and two double bonds. That is, any compound with formula HO(O=)C–(CH
2)
x–CH=CH–(CH
2)
y–CH=CH–(–CH
2)
z–H where x, y, and z can be zero or more, and x+y+z = 5 ; or HO(O=)C–(CH
2)
r–CH=C=CH–(CH
2)
s–H where r + s = 6. All these compounds have the formula C
10H
16O
2. A salt or ester of such an acid is called a decadienoate.












