| Grey matter | |
|---|---|
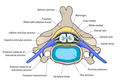
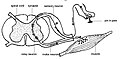
 The formation of the spinal nerve from the dorsal and ventral roots (with grey matter labelled at centre right). | |
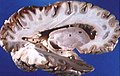
 Micrograph showing grey matter, with the characteristic neuronal cell bodies (dark shade of pink), and white matter with its characteristic fine meshwork-like appearance (left of image; lighter shade of pink). HPS stain. | |
| Details | |
| Identifiers | |
| Latin | substantia grisea |
| MeSH | D066128 |
| TA98 | A14.1.00.002 A14.1.02.020 A14.1.04.201 A14.1.05.201 A14.1.05.401 A14.1.06.301 |
| TA2 | 5365 |
| FMA | 67242 |
| Anatomical terminology | |
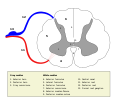
Grey matter (gray matter in American English) is a major component of the central nervous system, consisting of neuronal cell bodies, neuropil (dendrites and unmyelinated axons), glial cells (astrocytes and oligodendrocytes), synapses, and capillaries. Grey matter is distinguished from white matter in that it contains numerous cell bodies and relatively few myelinated axons, while white matter contains relatively few cell bodies and is composed chiefly of long-range myelinated axons. [1] The colour difference arises mainly from the whiteness of myelin. In living tissue, grey matter actually has a very light grey colour with yellowish or pinkish hues, which come from capillary blood vessels and neuronal cell bodies. [2]