Involvement in depression
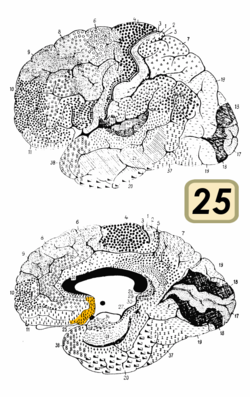
The subcallosal cingulate gyrus CG25 which consists of BA25 as well as parts of BA24 and BA32 has been implicated as playing an important role in major depression and has been the target of deep brain stimulation to treat the disorder. [6]
One study found that BA25 is metabolically overactive in treatment-resistant depression. [7] A different study found that metabolic hyperactivity in this area is associated with poor therapeutic response of persons with major depressive disorder to cognitive-behavioral therapy and venlafaxine. [8]
In 2005 Helen S. Mayberg and collaborators described how they successfully treated a number of depressed people—individuals virtually catatonic with depression despite years of talk therapy, drugs, and electroconvulsive therapy—with pacemaker-like electrodes (deep brain stimulation) in area 25. [9]
In 2013, Walker and Lawson conducted neurofeedback research with 183 subjects experiencing drug resistant depression. The neurofeedback specifically targeted Brodmann area 25. The treatment consisted of 6 neurofeedback sessions that were 20min long each over the course of 3 weeks. 1 year after the treatment 84% of subjects experienced over a 50% reduction in depression symptoms. 9% reported a 20-50% reduction in depression symptoms. 7% reported a 0-20% improvement. 1% initially reported positive effects, but had a depression relapse before the year follow up completed. There were no side effects or complications reported from the treatment. [10]
A study found that transcranial magnetic stimulation is more clinically effective treating depression when targeted specifically to Brodmann area 46, because this area has intrinsic functional connectivity (negative correlation) with area 25. [11]
Another study has found that the responses of area 25 to viewing sad stimuli are affected by cortisol. [12] This suggests that depression related changes in the activity in area 25 could be due to hypothalamic–pituitary–adrenal axis dysregulation.

Transcranial magnetic stimulation (TMS) is a noninvasive form of brain stimulation in which a changing magnetic field is used to induce an electric current at a specific area of the brain through electromagnetic induction. An electric pulse generator, or stimulator, is connected to a magnetic coil connected to the scalp. The stimulator generates a changing electric current within the coil which creates a varying magnetic field, inducing a current within a region in the brain itself.

Deep brain stimulation (DBS) is a surgical procedure that implants a neurostimulator and electrodes which sends electrical impulses to specified targets in the brain responsible for movement control. The treatment is designed for a range of movement disorders such as Parkinson's disease, essential tremor, and dystonia, as well as for certain neuropsychiatric conditions like obsessive-compulsive disorder (OCD) and epilepsy. The exact mechanisms of DBS are complex and not entirely clear, but it is known to modify brain activity in a structured way.

The cingulate cortex is a part of the brain situated in the medial aspect of the cerebral cortex. The cingulate cortex includes the entire cingulate gyrus, which lies immediately above the corpus callosum, and the continuation of this in the cingulate sulcus. The cingulate cortex is usually considered part of the limbic lobe.

In the human brain, the anterior cingulate cortex (ACC) is the frontal part of the cingulate cortex that resembles a "collar" surrounding the frontal part of the corpus callosum. It consists of Brodmann areas 24, 32, and 33.

A Brodmann area is a region of the cerebral cortex, in the human or other primate brain, defined by its cytoarchitecture, or histological structure and organization of cells. The concept was first introduced by the German anatomist Korbinian Brodmann in the early 20th century. Brodmann mapped the human brain based on the varied cellular structure across the cortex and identified 52 distinct regions, which he numbered 1 to 52. These regions, or Brodmann areas, correspond with diverse functions including sensation, motor control, and cognition.
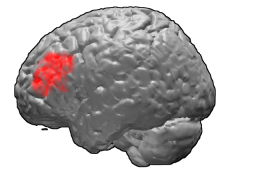
Brodmann area 46, or BA46, is part of the frontal cortex in the human brain. It is between BA10 and BA45.

Brodmann area 5 is one of Brodmann's cytoarchitectural defined regions of the brain. It is involved in somatosensory processing, movement and association, and is part of the posterior parietal cortex.
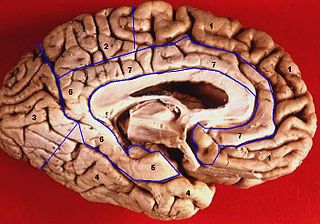
The limbic lobe is an arc-shaped cortical region of the limbic system, on the medial surface of each cerebral hemisphere of the mammalian brain, consisting of parts of the frontal, parietal and temporal lobes. The term is ambiguous, with some authors including the paraterminal gyrus, the subcallosal area, the cingulate gyrus, the parahippocampal gyrus, the dentate gyrus, the hippocampus and the subiculum; while the Terminologia Anatomica includes the cingulate sulcus, the cingulate gyrus, the isthmus of cingulate gyrus, the fasciolar gyrus, the parahippocampal gyrus, the parahippocampal sulcus, the dentate gyrus, the fimbrodentate sulcus, the fimbria of hippocampus, the collateral sulcus, and the rhinal sulcus, and omits the hippocampus.

The lobes of the brain are the major identifiable zones of the human cerebral cortex, and they comprise the surface of each hemisphere of the cerebrum. The two hemispheres are roughly symmetrical in structure, and are connected by the corpus callosum. They traditionally have been divided into four lobes, but are today considered as having six lobes each. The lobes are large areas that are anatomically distinguishable, and are also functionally distinct to some degree. Each lobe of the brain has numerous ridges, or gyri, and furrows, the sulci that constitute further subzones of the cortex. The expression "lobes of the brain" usually refers only to those of the cerebrum, not to the distinct areas of the cerebellum.
Treatment-resistant depression (TRD) is a term used in psychiatry to describe people with major depressive disorder (MDD) who do not respond adequately to a course of appropriate antidepressant medication within a certain time. Definitions of treatment-resistant depression vary, and they do not include a resistance to psychotherapy. Inadequate response has most commonly been defined as less than 50% reduction in depressive symptoms following treatment with at least one antidepressant medication, although definitions vary widely. Some other factors that may contribute to inadequate treatment are: a history of repeated or severe adverse childhood experiences, early discontinuation of treatment, insufficient dosage of medication, patient noncompliance, misdiagnosis, cognitive impairment, low income and other socio-economic variables, and concurrent medical conditions, including comorbid psychiatric disorders. Cases of treatment-resistant depression may also be referred to by which medications people with treatment-resistant depression are resistant to. In treatment-resistant depression adding further treatments such as psychotherapy, lithium, or aripiprazole is weakly supported as of 2019.

The posterior cingulate cortex (PCC) is the caudal part of the cingulate cortex, located posterior to the anterior cingulate cortex. This is the upper part of the "limbic lobe". The cingulate cortex is made up of an area around the midline of the brain. Surrounding areas include the retrosplenial cortex and the precuneus.
Helen S. Mayberg, is an American neurologist. Mayberg is known in particular for her work delineating abnormal brain function in patients with major depression using functional neuroimaging. This work led to the first pilot study of deep brain stimulation (DBS), a reversible method of selective modulation of a specific brain circuit, for patients with treatment-resistant depression. As of August 2019, she has published 211 original peer-reviewed articles, 31 books and book chapters, and acted as principal investigator on 24 research grants. Mayberg is coinventor with Andres Lozano of “Method for Treating Depression Mood Disorders and Anxiety Disorders using Neuromodulation,” US patent 2005/0033379A1. St. Jude Medical Neuromodulation licensed her intellectual property to develop Subcallosal Cingulate Deep Brain Stimulation for Treatment-Resistant Unipolar and Bipolar Depression for the treatment of severe depression. As of 2018, Mayberg holds positions as Professor of Neurology and Neurosurgery and Professor, Psychiatry and Neuroscience, both at Mount Sinai Medical School, and Professor of Psychiatry, Emory University; Emory University Hospital. Since 2018, she has served as Director, Nash Family Center for Advanced Circuit Therapeutics at the Icahn School of Medicine at Mount Sinai.

The subcallosal area is a small triangular field on the medial surface of the hemisphere in front of the subcallosal gyrus, from which it is separated by the posterior parolfactory sulcus; it is continuous below with the olfactory trigone, and above and in front with the cingulate gyrus; it is limited anteriorly by the anterior parolfactory sulcus.
Cordance, a measure of brain activity, is a quantitative electroencephalographic (QEEG) method, developed in Los Angeles in the 1990s. It combines complementary information from absolute and relative power of EEG spectra.
Subcortical ischemic depression, also known as vascular depression, is a medical condition most commonly seen in older people with major depressive disorder. Subcortical ischemic depression refers to vascular depression specifically due to lesions and restricted blood flow, known as ischemia, in certain parts of the brain. However, the disorder is typically described as vascular depression in the literature.
Management of depression is the treatment of depression that may involve a number of different therapies: medications, behavior therapy, psychotherapy, and medical devices.
Scientific studies have found that different brain areas show altered activity in humans with major depressive disorder (MDD), and this has encouraged advocates of various theories that seek to identify a biochemical origin of the disease, as opposed to theories that emphasize psychological or situational causes. Factors spanning these causative groups include nutritional deficiencies in magnesium, vitamin D, and tryptophan with situational origin but biological impact. Several theories concerning the biologically based cause of depression have been suggested over the years, including theories revolving around monoamine neurotransmitters, neuroplasticity, neurogenesis, inflammation and the circadian rhythm. Physical illnesses, including hypothyroidism and mitochondrial disease, can also trigger depressive symptoms.
Magnetic seizure therapy (MST) is a proposed form of electrotherapy and electrical brain stimulation. It is currently being investigated for the treatment of major depressive disorder, treatment-resistant depression (TRD), bipolar depression, schizophrenia and obsessive-compulsive disorder. MST is stated to work by inducing seizures via magnetic fields, in contrast to ECT which does so using alternating electric currents. Additionally, MST works in a more concentrated fashion than ECT, thus able to create a seizure with less of a total electric charge. In contrast to (r)TMS, the stimulation rates are higher resulting in more energy transfer. Currently it is thought that MST works in patients with major depressive disorder by activating the connection between the subgenual anterior cingulate cortex and the parietal cortex.

BrainsWay Ltd. is an international company that is engaged in the development of a medical device that uses H-coil for deep transcranial magnetic stimulation as a non-invasive treatment for depression, OCD, and smoking addiction. The company was founded in 2003 and has offices in the US and Jerusalem.

Abraham Zangen is an Israeli professor of neuroscience, head of the brain stimulation and behavior lab and chair of the psychobiology brain program at Ben-Gurion University of the Negev (BGU).