Related Research Articles

Deep brain stimulation (DBS) is a surgical procedure that implants a neurostimulator and electrodes which sends electrical impulses to specified targets in the brain responsible for movement control. The treatment is designed for a range of movement disorders such as Parkinson's disease, essential tremor, and dystonia, as well as for certain neuropsychiatric conditions like obsessive-compulsive disorder (OCD) and epilepsy. The exact mechanisms of DBS are complex and not entirely clear, but it is known to modify brain activity in a structured way.
Neurotechnology encompasses any method or electronic device which interfaces with the nervous system to monitor or modulate neural activity.

Vagus nerve stimulation (VNS) is a medical treatment that involves delivering electrical impulses to the vagus nerve. It is used as an add-on treatment for certain types of intractable epilepsy, cluster headaches, treatment-resistant depression and stroke rehabilitation.
Neurohacking is a subclass of biohacking, focused specifically on the brain. Neurohackers seek to better themselves or others by “hacking the brain” to improve reflexes, learn faster, or treat psychological disorders. The modern neurohacking movement has been around since the 1980s. However, herbal supplements have been used to increase brain function for hundreds of years. After a brief period marked by a lack of research in the area, neurohacking started regaining interest in the early 2000s. Currently, most neurohacking is performed via do-it-yourself (DIY) methods by in-home users.

Brodmann area 25 (BA25) is the subgenual area, area subgenualis or subgenual cingulate area in the cerebral cortex of the brain and delineated based on its cytoarchitectonic characteristics.
Treatment-resistant depression (TRD) is major depressive disorder in which an affected person does not respond adequately to at least two different antidepressant medications at an adequate dose and for an adequate duration. Inadequate response has most commonly been defined as less than 25% reduction in depressive symptoms following treatment with an antidepressant. Many clinicians and researchers question the construct validity and clinical utility of treatment-resistant depression as currently conceptualized.
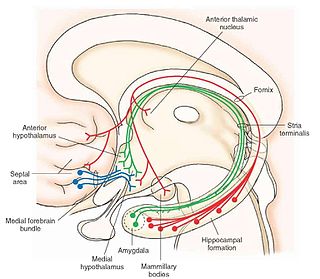
The medial forebrain bundle (MFB) is a neural pathway containing fibers from the basal olfactory regions, the periamygdaloid region and the septal nuclei, as well as fibers from brainstem regions, including the ventral tegmental area and nigrostriatal pathway.
Cordance, a measure of brain activity, is a quantitative electroencephalographic (QEEG) method, developed in Los Angeles in the 1990s. It combines complementary information from absolute and relative power of EEG spectra.
Responsive neurostimulation device is a medical device that senses changes in a person's body and uses neurostimulation to respond in the treatment of disease. The FDA has approved devices for use in the United States in the treatment of epileptic seizures and chronic pain conditions. Devices are being studied for use in the treatment of essential tremor, Parkinson's disease, Tourette's syndrome, depression, obesity, and post-traumatic stress disorder.
Management of depression is the treatment of depression that may involve a number of different therapies: medications, behavior therapy, psychotherapy, and medical devices.
Neuromodulation is "the alteration of nerve activity through targeted delivery of a stimulus, such as electrical stimulation or chemical agents, to specific neurological sites in the body". It is carried out to normalize – or modulate – nervous tissue function. Neuromodulation is an evolving therapy that can involve a range of electromagnetic stimuli such as a magnetic field (rTMS), an electric current, or a drug instilled directly in the subdural space. Emerging applications involve targeted introduction of genes or gene regulators and light (optogenetics), and by 2014, these had been at minimum demonstrated in mammalian models, or first-in-human data had been acquired. The most clinical experience has been with electrical stimulation.
Charles L. Raison is an American psychiatrist and professor of psychiatry at the University of Wisconsin-Madison School of Medicine and Public Health as well as the Mary Sue and Mike Shannon Chair for Healthy Minds, Children & Families and Professor with the School of Human Ecology in Madison, Wisconsin.

Abraham Zangen is an Israeli professor of neuroscience, head of the brain stimulation and behavior lab and chair of the psychobiology brain program at Ben-Gurion University of the Negev (BGU).
Ali R. Rezai is an Iranian-born American neurosurgeon and neuroscientist. His work and research has focused on neuromodulation treatments for patients with neurological and mental health conditions, including neuromodulation techniques such as deep brain stimulation (DBS) through brain chip implants to treat Parkinson's disease tremors, obsessive–compulsive disorder, Alzheimer's disease, traumatic brain injury, spinal cord injury, and addiction. Recent research since 2020 has focused on deep brain stimulation for addiction treatment, as well as focused ultrasound to treat tremor, addiction and Alzheimer's disease.
Non-invasive cerebellar stimulation is the application of non-invasive neurostimulation techniques on the cerebellum to modify its electrical activity. Techniques such as transcranial magnetic stimulation (TMS) or transcranial direct current stimulation (tDCS) can be used. The cerebellum is a high potential target for neuromodulation of neurological and psychiatric disorders due to the high density of neurons in its superficial layer, its electrical properties, and its participation in numerous closed-loop circuits involved in motor, cognitive, and emotional functions.

Meaghan Creed is a Canadian neuroscientist and associate professor of anesthesiology at Washington University in St. Louis. Creed has conducted research on understanding and optimizing deep brain stimulation in the basal ganglia for the treatment of neurological and psychiatric disorders. Her work has been recognized at the national and international level by Pfizer, the American Association for the Advancement of Science (AAAS), the Whitehall Foundation, Brain and Behavior Research Foundation and the Rita Allen Foundation.

Alberto Priori is an Italian neurologist, academic, and author. He is a Professor of Neurology at the University of Milan, Director of Neurology 1 Unit at San Paolo Hospital, and the Founder and Coordinator of Aldo Ravelli Center of the University of Milan. He also serves as President of the Neurophysiopatology Techniques Course, and Professor of Postgraduate Schools - Medicine, Healthcare, Dental Medicine at the same University.
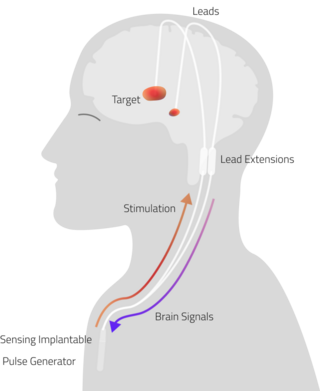
Adaptive Deep Brain Stimulation (aDBS), also known as Closed Loop Deep Brain stimulation (clDBS), is a neuro-modulatory technique currently under investigation for the treatment of neurodegenerative diseases.
Andres M. Lozano is a Spanish-Canadian neurosurgeon and scientist known for his work in Deep Brain Stimulation and MR guided Focused Ultrasound Surgery. He holds the Alan & Susan Hudson Cornerstone Chair in Neurosurgery at the University Health Network Toronto and is a University Professor at the University of Toronto. His work has been covered by major international news publications including BBC, Scientific American, The Independent, The Globe and Mail and NPR.
Interventional Psychiatry is a subspecialty within the field of psychiatry, focusing on the use of procedural and device-based treatments to manage mental health disorders, particularly those resistant to conventional therapies such as pharmacotherapy and psychotherapy. This field integrates neuromodulation methods with targeted pharmacological interventions, providing options for patients who have not responded to traditional treatments.
References
- ↑ "Witness: Ex-soldier suffered from impulse control issues". NBC News . Paducah, Kentucky. 2009-05-12. Retrieved 2018-02-12.
Ruben Gur, director of neuropsychology at the University of Pennsylvania School of Medicine, told jurors Tuesday that former Pfc. Steven Dale Green would be prone to acting inappropriately in chaotic situations because of the brain damage. Gur, testifying for the defense, said the brain damage likely was caused by several head injuries.
- ↑ Kevin Davis (2017). The Brain Defense: Murder in Manhattan and the Dawn of Neuroscience in America's Courtrooms. Penguin. ISBN 9780698183353 . Retrieved 2018-02-12.
- ↑ Filkowski, Megan M.; Mayberg, Helen; Holtzheimer, Paul E. (June 2016). "Considering Eligibility for Studies of Deep Brain Stimulation for Treatment-Resistant Depression Insights From a Clinical Trial in Unipolar and Bipolar Depression". Journal of ECT. 32 (2): 122–126. doi:10.1097/YCT.0000000000000281. PMC 4834065 . PMID 26479487 . Retrieved 2019-08-16.
- ↑ Holtzheimer PE III; Mayberg, H. S. (2010). "Deep Brain Stimulation for Treatment-Resistant Depression". The American Journal of Psychiatry. 167 (12): 1437–1444. doi:10.1176/appi.ajp.2010.10010141. PMC 4413473 . PMID 21131410.
- ↑ Holtzheimer, P. E.; Kelley, M. E.; Gross, R. E.; Filkowski, M. M.; Garlow, S. J.; Barrocas, A.; Wint, D.; Craighead, M. C.; Kozarsky, J.; Chismar, R.; Moreines, J. L.; Mewes, K.; Posse, P. R.; Gutman, D. A.; Mayberg, H. S. (January 2, 2012). "Subcallosal Cingulate Deep Brain Stimulation for Treatment-Resistant Unipolar and Bipolar Depression". Archives of General Psychiatry. 69 (2): 150–158. doi:10.1001/archgenpsychiatry.2011.1456. PMC 4423545 . PMID 22213770.
- ↑ "Helen Mayberg | Mount Sinai - New York". Mount Sinai Health System. Retrieved 2019-09-13.
- 1 2 Dobbs, David (2018-04-17). "Why a 'Lifesaving' Depression Treatment Didn't Pass Clinical Trials". The Atlantic. Retrieved 2019-09-13.
- ↑ "Helen Mayberg, M.D. | MindCORE". mindcore.sas.upenn.edu. Retrieved 2019-09-13.
- ↑ "William & Pudge Landau Lectureship | Office of Neuroscience Research | Washington University in St. Louis". neuroscienceresearch.wustl.edu. Retrieved 2019-09-13.
- 1 2 "NARSAD presents 2007 prizes for outstanding achievement in neuroscience and psychiatric research". EurekAlert!. Retrieved 2019-12-10.
- 1 2 "The 2010 Roche – Nature Medicine Translational Neuroscience Symposium : Spoonful of Medicine". blogs.nature.com. Archived from the original on 2019-12-10. Retrieved 2019-12-10.
- 1 2 "National Academy of Inventors Elects Lollar, Mayberg as Fellows > Publications > Hemophilia of Georgia". www.hog.org. Retrieved 2019-12-10.
- ↑ Witten, Mark (2004-04-12). "Mapping the Blues". The Walrus. Retrieved 2019-12-10.
- ↑ "Using Deep Brain Stimulation to Treat Depression". Discover Magazine. Retrieved 2019-12-10.
- ↑ Underwood, Emily (2013-11-01). "Short-Circuiting Depression". Science. 342 (6158): 548–551. Bibcode:2013Sci...342..548U. doi:10.1126/science.342.6158.548. ISSN 0036-8075. PMID 24179199.
- ↑ "Helen S. Mayberg". American Academy of Arts & Sciences. Retrieved 2019-12-10.
- ↑ "Helen S. Mayberg, M.D." Brain & Behavior Research Foundation. 2017-03-14. Retrieved 2019-12-10.
- ↑ "Biography of Helen Mayberg | Game Changers | School of Medicine, Emory University". med.emory.edu. Retrieved 2019-09-13.
- ↑ Shen, Helen H. (2019-03-12). "Core Concept: Can deep brain stimulation find success beyond Parkinson's disease?". Proceedings of the National Academy of Sciences. 116 (11): 4764–4766. Bibcode:2019PNAS..116.4764S. doi: 10.1073/pnas.1900442116 . ISSN 0027-8424. PMC 6421452 . PMID 30862742.
- ↑ "St. Jude Medical, BROADEN depression clinical trial failure, futility analysis, Helen Mayberg, deep brain stimulation (DBS) of Cg25, Parkinson's disease, FDA". www.neurotechreports.com. Retrieved 2019-12-10.
- ↑ "Changing Minds: Area 25". www.cbsnews.com. Retrieved 2019-12-10.
- ↑ "Full Page Reload". IEEE Spectrum: Technology, Engineering, and Science News. 18 April 2017. Retrieved 2019-12-10.
- ↑ Sendi, Mohammad S. E; Waters, Allison C; Tiruvadi, Vineet; Riva-Pose, Patricio; Crowell, Andrea; Isbaine, Faical; Gale, John.T; Choi, Ki Sueng; Gross, Robert. E; Mayberg, Helen. S; Mahmoudi, Babak (2021-11-03). "Intraoperative neural signals predict rapid antidepressant effects of deep brain stimulation". Translational Psychiatry. 11 (1): 551. doi:10.1038/s41398-021-01669-0. PMC 8563808 . PMID 34728599.
- ↑ Charles L. Raison, M. D. (2008-03-01). "Buddhists Meet Mind Scientists in Conference on Meditation and Depression". Psychiatric Times. Psychiatric Times Vol 25 No 3. 25. Retrieved 2019-12-11.
- ↑ Lama, The 14th Dalai (2019-12-10). "How Thinking Can Change the Brain". The 14th Dalai Lama. Retrieved 2019-12-11.
{{cite web}}: CS1 maint: numeric names: authors list (link) - ↑ "How meditating may help your brain". Archived from the original on November 23, 2011. Retrieved 2019-12-11.
- ↑ "When It Comes to Issues of Identity and Authenticity in DBS, Let Patients Have a Voice". www.sfn.org. Retrieved 2019-12-11.
- 1 2 "Leadership". www.neuroethicssociety.org. Retrieved 13 February 2018.
- ↑ "Transcript, Meeting 18, Session 3 | Presidential Commission for the Study of Bioethical Issues". bioethicsarchive.georgetown.edu. Retrieved 2019-12-11.
- ↑ Magazine, Eryn Brown,Knowable. "Is "Neurolaw" Coming Soon to a Courtroom Near You?". Scientific American. Retrieved 2019-12-11.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ↑ Rosen, Jeffrey (2007-03-11). "Neuroscience - Law - The Brain on the Stand - Jeffrey Rosen". The New York Times. ISSN 0362-4331 . Retrieved 2019-12-11.
- ↑ Hughes, Virginia (2010-03-01). "Science in court: Head case". Nature. 464 (7287): 340–342. doi: 10.1038/464340a . ISSN 1476-4687. PMID 20237536.
- ↑ Raine, Adrian (2013-04-26). "The Criminal Mind". Wall Street Journal. ISSN 0099-9660 . Retrieved 2019-12-11.
- ↑ "Criminologist Believes Violent Behavior Is Biological". NPR.org. Retrieved 2019-12-11.
- ↑ "Helen S. Mayberg, M.D Biography". Hope For Depression Research Institute. Retrieved 2019-08-19.
- ↑ "Two Emory faculty elected to American Academy of Arts and Sciences". Emory News Center. April 11, 2017. Retrieved 2019-08-19.
- ↑ "V. Sagar Sethi, M.D. Mental Health Research Award". www.ncpsychiatry.org. Retrieved 2019-08-20.
- ↑ "Helen S. Mayberg". National Academy of Medicine. Retrieved 2020-07-20.
- ↑ "National Academy of Inventors Elects Lollar, Mayberg as Fellows > Publications > Hemophilia of Georgia". Hemophilia of Georgia. Retrieved 2019-08-20.
- ↑ "Society of Biological Psychiatry Gold Medal Award" . Retrieved 2019-08-20.
- ↑ "Society of Biological Psychiatry Gold Medal Award | Society of Biological Psychiatry" . Retrieved 2019-12-10.
- ↑ "Helen Mayberg Wins Psychiatry Award". Dana Foundation. 2013-04-11. Retrieved 2019-12-10.
- 1 2 3 4 5 6 7 8 9 "Google Scholar" . Retrieved 16 August 2019.