
Within a nervous system, a neuron, neurone, or nerve cell is an electrically excitable cell that fires electric signals called action potentials across a neural network. Neurons communicate with other cells via synapses, which are specialized connections that commonly use minute amounts of chemical neurotransmitters to pass the electric signal from the presynaptic neuron to the target cell through the synaptic gap.
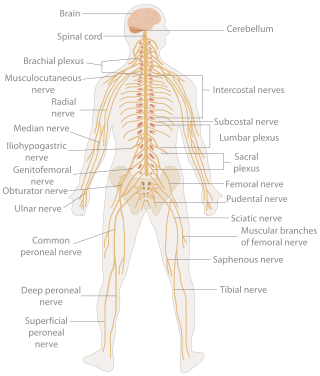
In biology, the nervous system is the highly complex part of an animal that coordinates its actions and sensory information by transmitting signals to and from different parts of its body. The nervous system detects environmental changes that impact the body, then works in tandem with the endocrine system to respond to such events. Nervous tissue first arose in wormlike organisms about 550 to 600 million years ago. In vertebrates, it consists of two main parts, the central nervous system (CNS) and the peripheral nervous system (PNS). The CNS consists of the brain and spinal cord. The PNS consists mainly of nerves, which are enclosed bundles of the long fibers, or axons, that connect the CNS to every other part of the body. Nerves that transmit signals from the brain are called motor nerves (efferent), while those nerves that transmit information from the body to the CNS are called sensory nerves (afferent). The PNS is divided into two separate subsystems, the somatic and autonomic, nervous systems. The autonomic nervous system is further subdivided into the sympathetic, parasympathetic and enteric nervous systems. The sympathetic nervous system is activated in cases of emergencies to mobilize energy, while the parasympathetic nervous system is activated when organisms are in a relaxed state. The enteric nervous system functions to control the gastrointestinal system. Nerves that exit from the brain are called cranial nerves while those exiting from the spinal cord are called spinal nerves.
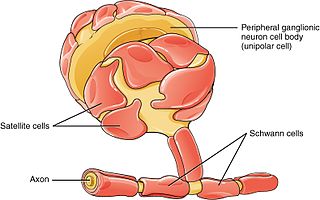
Schwann cells or neurolemmocytes are the principal glia of the peripheral nervous system (PNS). Glial cells function to support neurons and in the PNS, also include satellite cells, olfactory ensheathing cells, enteric glia and glia that reside at sensory nerve endings, such as the Pacinian corpuscle. The two types of Schwann cells are myelinating and nonmyelinating. Myelinating Schwann cells wrap around axons of motor and sensory neurons to form the myelin sheath. The Schwann cell promoter is present in the downstream region of the human dystrophin gene that gives shortened transcript that are again synthesized in a tissue-specific manner.

The autonomic nervous system (ANS), sometimes called the visceral nervous system and formerly the vegetative nervous system, is a division of the nervous system that operates internal organs, smooth muscle and glands. The autonomic nervous system is a control system that acts largely unconsciously and regulates bodily functions, such as the heart rate, its force of contraction, digestion, respiratory rate, pupillary response, urination, and sexual arousal. This system is the primary mechanism in control of the fight-or-flight response.
Nervous tissue, also called neural tissue, is the main tissue component of the nervous system. The nervous system regulates and controls body functions and activity. It consists of two parts: the central nervous system (CNS) comprising the brain and spinal cord, and the peripheral nervous system (PNS) comprising the branching peripheral nerves. It is composed of neurons, also known as nerve cells, which receive and transmit impulses, and neuroglia, also known as glial cells or glia, which assist the propagation of the nerve impulse as well as provide nutrients to the neurons.

Glia, also called glial cells (gliocytes) or neuroglia, are non-neuronal cells in the central nervous system and the peripheral nervous system that do not produce electrical impulses. The neuroglia make up more than one half the volume of neural tissue in the human body. They maintain homeostasis, form myelin in the peripheral nervous system, and provide support and protection for neurons. In the central nervous system, glial cells include oligodendrocytes, astrocytes, ependymal cells and microglia, and in the peripheral nervous system they include Schwann cells and satellite cells.

Astrocytes, also known collectively as astroglia, are characteristic star-shaped glial cells in the brain and spinal cord. They perform many functions, including biochemical control of endothelial cells that form the blood–brain barrier, provision of nutrients to the nervous tissue, maintenance of extracellular ion balance, regulation of cerebral blood flow, and a role in the repair and scarring process of the brain and spinal cord following infection and traumatic injuries. The proportion of astrocytes in the brain is not well defined; depending on the counting technique used, studies have found that the astrocyte proportion varies by region and ranges from 20% to around 40% of all glia. Another study reports that astrocytes are the most numerous cell type in the brain. Astrocytes are the major source of cholesterol in the central nervous system. Apolipoprotein E transports cholesterol from astrocytes to neurons and other glial cells, regulating cell signaling in the brain. Astrocytes in humans are more than twenty times larger than in rodent brains, and make contact with more than ten times the number of synapses.

Astrogliosis is an abnormal increase in the number of astrocytes due to the destruction of nearby neurons from central nervous system (CNS) trauma, infection, ischemia, stroke, autoimmune responses or neurodegenerative disease. In healthy neural tissue, astrocytes play critical roles in energy provision, regulation of blood flow, homeostasis of extracellular fluid, homeostasis of ions and transmitters, regulation of synapse function and synaptic remodeling. Astrogliosis changes the molecular expression and morphology of astrocytes, in response to infection for example, in severe cases causing glial scar formation that may inhibit axon regeneration.
Oligodendrocyte progenitor cells (OPCs), also known as oligodendrocyte precursor cells, NG2-glia, O2A cells, or polydendrocytes, are a subtype of glia in the central nervous system named for their essential role as precursors to oligodendrocytes. They are typically identified in the human by co-expression of PDGFRA and CSPG4.

Glutamate receptors are synaptic and non synaptic receptors located primarily on the membranes of neuronal and glial cells. Glutamate is abundant in the human body, but particularly in the nervous system and especially prominent in the human brain where it is the body's most prominent neurotransmitter, the brain's main excitatory neurotransmitter, and also the precursor for GABA, the brain's main inhibitory neurotransmitter. Glutamate receptors are responsible for the glutamate-mediated postsynaptic excitation of neural cells, and are important for neural communication, memory formation, learning, and regulation.

The neuroimmune system is a system of structures and processes involving the biochemical and electrophysiological interactions between the nervous system and immune system which protect neurons from pathogens. It serves to protect neurons against disease by maintaining selectively permeable barriers, mediating neuroinflammation and wound healing in damaged neurons, and mobilizing host defenses against pathogens.
Glutamate transporters are a family of neurotransmitter transporter proteins that move glutamate – the principal excitatory neurotransmitter – across a membrane. The family of glutamate transporters is composed of two primary subclasses: the excitatory amino acid transporter (EAAT) family and vesicular glutamate transporter (VGLUT) family. In the brain, EAATs remove glutamate from the synaptic cleft and extrasynaptic sites via glutamate reuptake into glial cells and neurons, while VGLUTs move glutamate from the cell cytoplasm into synaptic vesicles. Glutamate transporters also transport aspartate and are present in virtually all peripheral tissues, including the heart, liver, testes, and bone. They exhibit stereoselectivity for L-glutamate but transport both L-aspartate and D-aspartate.
Gliosis is a nonspecific reactive change of glial cells in response to damage to the central nervous system (CNS). In most cases, gliosis involves the proliferation or hypertrophy of several different types of glial cells, including astrocytes, microglia, and oligodendrocytes. In its most extreme form, the proliferation associated with gliosis leads to the formation of a glial scar.

Radial glial cells, or radial glial progenitor cells (RGPs), are bipolar-shaped progenitor cells that are responsible for producing all of the neurons in the cerebral cortex. RGPs also produce certain lineages of glia, including astrocytes and oligodendrocytes. Their cell bodies (somata) reside in the embryonic ventricular zone, which lies next to the developing ventricular system.

The glia limitans, or the glial limiting membrane, is a thin barrier of astrocyte foot processes associated with the parenchymal basal lamina surrounding the brain and spinal cord. It is the outermost layer of neural tissue, and among its responsibilities is the prevention of the over-migration of neurons and neuroglia, the supporting cells of the nervous system, into the meninges. The glia limitans also plays an important role in regulating the movement of small molecules and cells into the brain tissue by working in concert with other components of the central nervous system (CNS) such as the blood–brain barrier (BBB).
Gliotransmitters are chemicals released from glial cells that facilitate neuronal communication between neurons and other glial cells. They are usually induced from Ca2+ signaling, although recent research has questioned the role of Ca2+ in gliotransmitters and may require a revision of the relevance of gliotransmitters in neuronal signalling in general.
In biochemistry, the glutamate–glutamine cycle is a cyclic metabolic pathway which maintains an adequate supply of the neurotransmitter glutamate in the central nervous system. Neurons are unable to synthesize either the excitatory neurotransmitter glutamate, or the inhibitory GABA from glucose. Discoveries of glutamate and glutamine pools within intercellular compartments led to suggestions of the glutamate–glutamine cycle working between neurons and astrocytes. The glutamate/GABA–glutamine cycle is a metabolic pathway that describes the release of either glutamate or GABA from neurons which is then taken up into astrocytes. In return, astrocytes release glutamine to be taken up into neurons for use as a precursor to the synthesis of either glutamate or GABA.
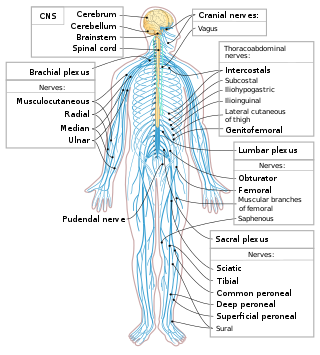
The following diagram is provided as an overview of and topical guide to the human nervous system:

Tripartite synapse refers to the functional integration and physical proximity of:

Alexei Verkhratsky, sometimes spelled Alexej, is a professor of neurophysiology at the University of Manchester best known for his research on the physiology and pathophysiology of neuroglia, calcium signalling, and brain ageing. He is an elected member and vice-president of Academia Europaea, of the German National Academy of Sciences Leopoldina, of the Real Academia Nacional de Farmacia (Spain), of the Slovenian Academy of Sciences and Arts, of Polish Academy of Sciences, and Dana Alliance for Brain Initiatives, among others. Since 2010, he is a Ikerbasque Research Professor and from 2012 he is deputy director of the Achucarro Basque Center for Neuroscience in Bilbao. He is a distinguished professor at Jinan University, China Medical University of Shenyang, and Chengdu University of Traditional Chinese Medicine and is an editor-in-chief of Cell Calcium, receiving editor for Cell Death and Disease, and Acta Physiologica and member of editorial board of many academic journals.


















