Related Research Articles
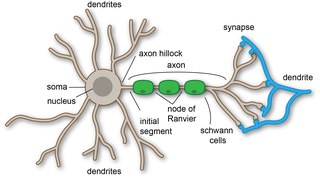
A dendrite or dendron is a branched cytoplasmic process that extends from a nerve cell that propagates the electrochemical stimulation received from other neural cells to the cell body, or soma, of the neuron from which the dendrites project. Electrical stimulation is transmitted onto dendrites by upstream neurons via synapses which are located at various points throughout the dendritic tree.

The hippocampus, also hippocampus proper, is a major component of the brain of humans and many other vertebrates. In the human brain the hippocampus, the dentate gyrus, and the subiculum are the components of the hippocampal formation located in the limbic system. The hippocampus plays important roles in the consolidation of information from short-term memory to long-term memory, and in spatial memory that enables navigation. In humans, and other primates the hippocampus is located in the archicortex, one of the three regions of allocortex, in each hemisphere with neural projections to the neocortex. The hippocampus, as the medial pallium, is a structure found in all vertebrates.
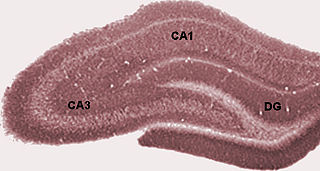
The dentate gyrus (DG) is part of the hippocampal formation in the temporal lobe of the brain, which also includes the hippocampus and the subiculum. The dentate gyrus is part of the hippocampal trisynaptic circuit and is thought to contribute to the formation of new episodic memories, the spontaneous exploration of novel environments and other functions. The dentate gyrus has toothlike projections from which it is named.
An inhibitory postsynaptic potential (IPSP) is a kind of synaptic potential that makes a postsynaptic neuron less likely to generate an action potential. The opposite of an inhibitory postsynaptic potential is an excitatory postsynaptic potential (EPSP), which is a synaptic potential that makes a postsynaptic neuron more likely to generate an action potential. IPSPs can take place at all chemical synapses, which use the secretion of neurotransmitters to create cell-to-cell signalling. EPSPs and IPSPs compete with each other at numerous synapses of a neuron. This determines whether an action potential occurring at the presynaptic terminal produces an action potential at the postsynaptic membrane. Some common neurotransmitters involved in IPSPs are GABA and glycine.

In neuroscience, an excitatory postsynaptic potential (EPSP) is a postsynaptic potential that makes the postsynaptic neuron more likely to fire an action potential. This temporary depolarization of postsynaptic membrane potential, caused by the flow of positively charged ions into the postsynaptic cell, is a result of opening ligand-gated ion channels. These are the opposite of inhibitory postsynaptic potentials (IPSPs), which usually result from the flow of negative ions into the cell or positive ions out of the cell. EPSPs can also result from a decrease in outgoing positive charges, while IPSPs are sometimes caused by an increase in positive charge outflow. The flow of ions that causes an EPSP is an excitatory postsynaptic current (EPSC).
In neurophysiology, long-term depression (LTD) is an activity-dependent reduction in the efficacy of neuronal synapses lasting hours or longer following a long patterned stimulus. LTD occurs in many areas of the CNS with varying mechanisms depending upon brain region and developmental progress.

Pyramidal cells, or pyramidal neurons, are a type of multipolar neuron found in areas of the brain including the cerebral cortex, the hippocampus, and the amygdala. Pyramidal cells are the primary excitation units of the mammalian prefrontal cortex and the corticospinal tract. One of the main structural features of the pyramidal neuron is the conic shaped soma, or cell body, after which the neuron is named. Other key structural features of the pyramidal cell are a single axon, a large apical dendrite, multiple basal dendrites, and the presence of dendritic spines.

Basket cells are inhibitory GABAergic interneurons of the brain, found throughout different regions of the cortex and cerebellum.
Schaffer collaterals are axon collaterals given off by CA3 pyramidal cells in the hippocampus. These collaterals project to area CA1 of the hippocampus and are an integral part of memory formation and the emotional network of the Papez circuit, and of the hippocampal trisynaptic loop. It is one of the most studied synapses in the world and named after the Hungarian anatomist-neurologist Károly Schaffer.
A basal dendrite is a dendrite that emerges from the base of a pyramidal cell that receives information from nearby neurons and passes it to the soma, or cell body. Due to their direct attachment to the cell body itself, basal dendrites are able to deliver strong depolarizing currents and therefore have a strong effect on action potential output in neurons. The physical characteristics of basal dendrites vary based on their location and species that they are found in. For example, the basal dendrites of humans are overall found to be the most intricate and spine-dense, as compared to other species such as Macaques. It is also observed that basal dendrites of the prefrontal cortex are larger and more complex in comparison to the smaller and simpler dendrites that can be seen within the visual cortex. Basal dendrites are capable of vast amounts of analog computing, which is responsible for many of the different nonlinear responses of modulating information in the neocortex. Basal dendrites additionally exist in dentate granule cells for a limited time before removal via regulatory factors. This removal usually occurs before the cell reaches adulthood, and is thought to be regulated through both intracellular and extracellular signals. Basal dendrites are part of the more overarching dendritic tree present on pyramidal neurons. They, along with apical dendrites, make up the part of the neuron that receives most of the electrical signaling. Basal dendrites have been found to be involved mostly in neocortical information processing.

In the brain, the perforant path or perforant pathway provides a connectional route from the entorhinal cortex to all fields of the hippocampal formation, including the dentate gyrus, all CA fields, and the subiculum.
The stratum lucidum of the hippocampus is a layer of the hippocampus between the stratum pyramidale and the stratum radiatum. It is the tract of the mossy fiber projections, both inhibitory and excitatory from the granule cells of the dentate gyrus. One mossy fiber may make up to 37 connections to a single pyramidal cell, and innervate around 12 pyramidal cells on top of that. Any given pyramidal cell in the stratum lucidum may get input from as many as 50 granule cells.

In the hippocampus, the mossy fiber pathway consists of unmyelinated axons projecting from granule cells in the dentate gyrus that terminate on modulatory hilar mossy cells and in Cornu Ammonis area 3 (CA3), a region involved in encoding short-term memory. These axons were first described as mossy fibers by Santiago Ramón y Cajal as they displayed varicosities along their lengths that gave them a mossy appearance.
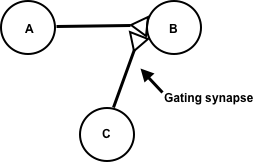
Synaptic gating is the ability of neural circuits to gate inputs by either suppressing or facilitating specific synaptic activity. Selective inhibition of certain synapses has been studied thoroughly, and recent studies have supported the existence of permissively gated synaptic transmission. In general, synaptic gating involves a mechanism of central control over neuronal output. It includes a sort of gatekeeper neuron, which has the ability to influence transmission of information to selected targets independently of the parts of the synapse upon which it exerts its action.
The trisynaptic circuit or trisynaptic loop is a relay of synaptic transmission in the hippocampus. The trisynaptic circuit is a neural circuit in the hippocampus, which is made up of three major cell groups: granule cells in the dentate gyrus, pyramidal neurons in CA3, and pyramidal neurons in CA1. The hippocampal relay involves 3 main regions within the hippocampus which are classified according to their cell type and projection fibers. The first projection of the hippocampus occurs between the entorhinal cortex (EC) and the dentate gyrus (DG). The entorhinal cortex transmits its signals from the parahippocampal gyrus to the dentate gyrus via granule cell fibers known collectively as the perforant path. The dentate gyrus then synapses on pyramidal cells in CA3 via mossy cell fibers. CA3 then fires to CA1 via Schaffer collaterals which synapse in the subiculum and are carried out through the fornix. Collectively the dentate gyrus, CA1 and CA3 of the hippocampus compose the trisynaptic loop.

Hippocampus anatomy describes the physical aspects and properties of the hippocampus, a neural structure in the medial temporal lobe of the brain. It has a distinctive, curved shape that has been likened to the sea-horse monster of Greek mythology and the ram's horns of Amun in Egyptian mythology. This general layout holds across the full range of mammalian species, from hedgehog to human, although the details vary. For example, in the rat, the two hippocampi look similar to a pair of bananas, joined at the stems. In the human and other primates, the portion of the hippocampus near the base of the temporal lobe is much broader than the part at the top. Due to the three-dimensional curvature of this structure, two-dimensional sections such as shown are commonly seen. Neuroimaging pictures can show a number of different shapes, depending on the angle and location of the cut.

In neurophysiology, a dendritic spike refers to an action potential generated in the dendrite of a neuron. Dendrites are branched extensions of a neuron. They receive electrical signals emitted from projecting neurons and transfer these signals to the cell body, or soma. Dendritic signaling has traditionally been viewed as a passive mode of electrical signaling. Unlike its axon counterpart which can generate signals through action potentials, dendrites were believed to only have the ability to propagate electrical signals by physical means: changes in conductance, length, cross sectional area, etc. However, the existence of dendritic spikes was proposed and demonstrated by W. Alden Spencer, Eric Kandel, Rodolfo Llinás and coworkers in the 1960s and a large body of evidence now makes it clear that dendrites are active neuronal structures. Dendrites contain voltage-gated ion channels giving them the ability to generate action potentials. Dendritic spikes have been recorded in numerous types of neurons in the brain and are thought to have great implications in neuronal communication, memory, and learning. They are one of the major factors in long-term potentiation.

The name granule cell has been used for a number of different types of neurons whose only common feature is that they all have very small cell bodies. Granule cells are found within the granular layer of the cerebellum, the dentate gyrus of the hippocampus, the superficial layer of the dorsal cochlear nucleus, the olfactory bulb, and the cerebral cortex.

Sharp waves and ripples (SPW-R), also called sharp wave ripples (SWR), are oscillatory patterns produced by extremely synchronized activity of neurons in the mammalian hippocampus and neighboring regions which occur spontaneously in idle waking states or during NREM sleep. They can be observed with a variety of electrophysiological methods such as field recordings or EEG. They are composed of large amplitude sharp waves in local field potential and produced by thousands of neurons firing together within a 30–100 ms window. Within this broad time window, pyramidal cells fire only at specific times set by fast spiking GABAergic interneurons. The fast rhythm of inhibition synchronizes the firing of active pyramidal cells, each of which only fires one or two action potentials exactly between the inhibitory peaks, collectively generating the ripple pattern. SWRs have been extensively characterized by György Buzsáki and have been shown to be involved in memory consolidation in NREM sleep. Neuronal firing sequences acquired during wakefulness are replayed during SWRs.
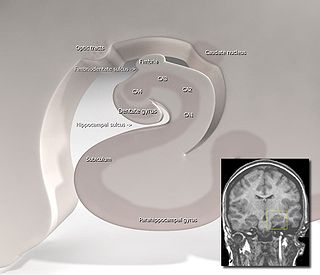
The hippocampal subfields are regions in the hippocampal formation that include the four subregions that make up the structure of the hippocampus, and the dentate gyrus, the presubiculum, and the subiculum. The subfields CA1, CA2, CA3, and CA4 use the initials of cornu ammonis, an earlier name of the hippocampus.
References
- 1 2 3 4 5 Greenstein BGaA. Color Atlas of Neuroscience: Neuroanatomy and Neurophysiology. Stuttgart, New York: Thieme; 2000
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73 74 75 76 77 Jerome Engel TAP, ed. Epilepsy: A Comprehensive Textbook in Three Volumes. Philadelphia, PA: Lippincott Williams & Wilkins; 2008
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 Cline HT. Dendritic arbor development and synaptogenesis. Current Opinion in Neurobiology 2001; 11: 118–126
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 Henze DA CW, Barrioneuvo G. Dendritic Morphology and its effects on the amplitude and rise-time of synaptic signals in hippocampal CA3 pyramidal cells. Journal of Comparative Neurology. 1996;369:331–344.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 CL W. Dendritic Reorganization in Pyramidal Neurons in Medial Prefrontal Cortex after Chronic Corticosterone Administration. Journal of Neurobiology. 2001;49:245–253.
- 1 2 3 Jibiki I MK, Ohtani T, et al. Dendritic Potential in Direct Cortical Responses and Seizure Activity. Folia Psychiatrica et Neurologica. 1978;32(3):329–337
- ↑ Bradshaw KD EN, Bliss TVP. SHORT COMMUNICATION: A role for dendritic protein synthesis in hippocampal late LTP. European Journal of Neuroscience. 2003;18:3150–3152
- 1 2 3 4 Lothman EW BE, and Stringer JL. Functional Anatomy of Hippocampal Seizures. Progress in Neurobiology. 1991;37:1–82.
- 1 2 3 4 5 6 7 8 Mathews, Gregory. Telephone Interview.11/19/08.
- 1 2 3 4 5 6 Dale Purves GJA, David Fitzpatrick, William C. Hall, Anthony-Samuel LaMantia, James O. McNamara, and S. Mark Williams, eds. Neuroscience: Third Edition. Sunderland, MA: Sinauer Associates, Inc.; 2004
- 1 2 3 Smith CUM. Elements of Molecular Neurobiology. 3rd ed. Chichester, West Sussex England: John Wiley & Sons Ltd; 2002.
- ↑ Bender RA BA, and Baram TZ. Neuronal Activity Influences the sub-cellular distribution of hyperpolarization-activated cation channels in hippocampal neurons. Epilepsia. 2005;46(supplement 8):92
- ↑ Groc L PZ, Gustafsson B, et al. In vivo blockade of neural activity alters dendritic development of neonatal CA1 pyramidal cells. European Journal of Neuroscience. 2002;16:1931–1938.
- 1 2 3 Anderson P MR, Amaral D, Bliss T, and O'Keefe J, ed. The Hippocampus Book: Oxford University Press.
- 1 2 3 4 Murmu MS SS, Biala Y, et al. Changes of spine density and dendritic complexity in the prefrontal cortex in offspring of mothers exposed to stress during pregnancy. European Journal of Neuroscience. 2006;24:1477–1487.
- 1 2 McKittrick CR MA, Blanchard DC, et al. Chronic Social Stress Reduces Dendritic arbors in CA3 of Hippocampus and Decreases Binding to Serotonin Transporter Sites. Synapse. 2000;36:85-942006;24:1477-1487.
- 1 2 3 4 5 Reith MEA, ed. Cerebral Signal Transduction: From First to Fourth Messengers. Totowa, NJ: Humana Press, Inc.; 2000.
- ↑ Haberland C. Clinical Neuropathology: Text and Color Atlas. New York, NY: Demos Medical Publishing, LLC; 2007.
- 1 2 3 4 Buccoliero A BJ, and Futerman AH. The role of sphingolipids in neuronal development: lessons from models of sphingolipid storage diseases. Neurochemical Research. 2002;27(7/8):565-574
- ↑ Dudek FE RM. Current opinions in clinical science: calcium currents burst back: a possible role for dendrites in eliptogenesis. Epilepsy Currents. 2007;7(5):140–141.
- 1 2 3 4 5 Benes FM TM, and Kostoulakos P. GluR5,6,7 Subunit Immunoreactivity on Apical Pyramidal Cell Dendrites in Hippocampus of Schizophrenics and Manic Depressives. Hippocampus. 2001;11:482–491.
- 1 2 Wong M. Modulation of dendritic spines in epilepsy: cellular mechanisms and functional implications. Epilepsy & Behavior. 2005;7:569–577.
- 1 2 3 4 5 6 7 8 9 Zani A PA, ed. The Cognitive Electrophysiology of Mind and Brain; 2002