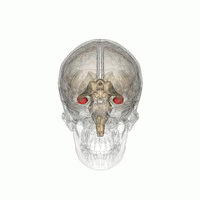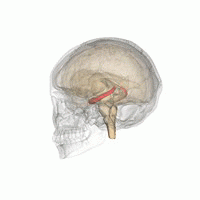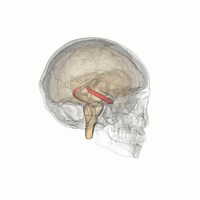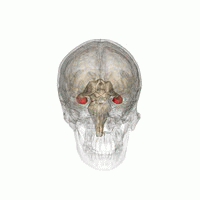
The hippocampus is a major component of the brain of humans and other vertebrates. Humans and other mammals have two hippocampi, one in each side of the brain. The hippocampus is part of the limbic system, and plays important roles in the consolidation of information from short-term memory to long-term memory, and in spatial memory that enables navigation. The hippocampus is located in the allocortex, with neural projections into the neocortex, in humans as well as other primates. The hippocampus, as the medial pallium, is a structure found in all vertebrates. In humans, it contains two main interlocking parts: the hippocampus proper, and the dentate gyrus.
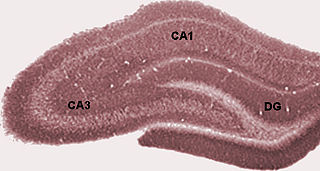
The dentate gyrus (DG) is part of the hippocampal formation in the temporal lobe of the brain, which also includes the hippocampus and the subiculum. The dentate gyrus is part of the hippocampal trisynaptic circuit and is thought to contribute to the formation of new episodic memories, the spontaneous exploration of novel environments and other functions.
An inhibitory postsynaptic potential (IPSP) is a kind of synaptic potential that makes a postsynaptic neuron less likely to generate an action potential. IPSPs were first investigated in motorneurons by David P. C. Lloyd, John Eccles and Rodolfo Llinás in the 1950s and 1960s. The opposite of an inhibitory postsynaptic potential is an excitatory postsynaptic potential (EPSP), which is a synaptic potential that makes a postsynaptic neuron more likely to generate an action potential. IPSPs can take place at all chemical synapses, which use the secretion of neurotransmitters to create cell to cell signalling. Inhibitory presynaptic neurons release neurotransmitters that then bind to the postsynaptic receptors; this induces a change in the permeability of the postsynaptic neuronal membrane to particular ions. An electric current that changes the postsynaptic membrane potential to create a more negative postsynaptic potential is generated, i.e. the postsynaptic membrane potential becomes more negative than the resting membrane potential, and this is called hyperpolarisation. To generate an action potential, the postsynaptic membrane must depolarize—the membrane potential must reach a voltage threshold more positive than the resting membrane potential. Therefore, hyperpolarisation of the postsynaptic membrane makes it less likely for depolarisation to sufficiently occur to generate an action potential in the postsynaptic neurone.

In neuroscience, an excitatory postsynaptic potential (EPSP) is a postsynaptic potential that makes the postsynaptic neuron more likely to fire an action potential. This temporary depolarization of postsynaptic membrane potential, caused by the flow of positively charged ions into the postsynaptic cell, is a result of opening ligand-gated ion channels. These are the opposite of inhibitory postsynaptic potentials (IPSPs), which usually result from the flow of negative ions into the cell or positive ions out of the cell. EPSPs can also result from a decrease in outgoing positive charges, while IPSPs are sometimes caused by an increase in positive charge outflow. The flow of ions that causes an EPSP is an excitatory postsynaptic current (EPSC).
In neuroanatomy, a neural pathway is the connection formed by axons that project from neurons to make synapses onto neurons in another location, to enable neurotransmission. Neurons are connected by a single axon, or by a bundle of axons known as a nerve tract, or fasciculus. Shorter neural pathways are found within grey matter in the brain, whereas longer projections, made up of myelinated axons, constitute white matter.

Pyramidal cells, or pyramidal neurons, are a type of multipolar neuron found in areas of the brain including the cerebral cortex, the hippocampus, and the amygdala. Pyramidal cells are the primary excitation units of the mammalian prefrontal cortex and the corticospinal tract. Pyramidal neurons are also one of two cell types where the characteristic sign, Negri bodies, are found in post-mortem rabies infection. Pyramidal neurons were first discovered and studied by Santiago Ramón y Cajal. Since then, studies on pyramidal neurons have focused on topics ranging from neuroplasticity to cognition.

Basket cells are inhibitory GABAergic interneurons of the brain, found throughout different regions of the cortex and cerebellum.
Schaffer collaterals are axon collaterals given off by CA3 pyramidal cells in the hippocampus. These collaterals project to area CA1 of the hippocampus and are an integral part of memory formation and the emotional network of the Papez circuit, and of the hippocampal trisynaptic loop. It is one of the most studied synapses in the world and named after the Hungarian anatomist-neurologist Károly Schaffer.
An apical dendrite is a dendrite that emerges from the apex of a pyramidal cell. Apical dendrites are one of two primary categories of dendrites, and they distinguish the pyramidal cells from spiny stellate cells in the cortices. Pyramidal cells are found in the prefrontal cortex, the hippocampus, the entorhinal cortex, the olfactory cortex, and other areas. Dendrite arbors formed by apical dendrites are the means by which synaptic inputs into a cell are integrated. The apical dendrites in these regions contribute significantly to memory, learning, and sensory associations by modulating the excitatory and inhibitory signals received by the pyramidal cells.

In neuroscience, Golgi cells are the most abundant inhibitory interneurons found within the granular layer of the cerebellum. Golgi cells can be found in the granular layer at various layers. The Golgi cell is essential for controlling the activity of the granular layer. They were first identified as inhibitory in 1964. It was also the first example of an inhibitory feedback network in which the inhibitory interneuron was identified anatomically. Golgi cells produce a wide lateral inhibition that reaches beyond the afferent synaptic field and inhibit granule cells via feedforward and feedback inhibitory loops. These cells synapse onto the dendrite of granule cells and unipolar brush cells. They receive excitatory input from mossy fibres, also synapsing on granule cells, and parallel fibers, which are long granule cell axons. Thereby this circuitry allows for feed-forward and feed-back inhibition of granule cells.

In the brain, the perforant path or perforant pathway provides a connectional route from the entorhinal cortex to all fields of the hippocampal formation, including the dentate gyrus, all CA fields, and the subiculum.

The median raphe nucleus, also known as the nucleus raphes medianus (NRM) or superior central nucleus, is a brain region composed of polygonal, fusiform, and piriform neurons, which exists rostral to the nucleus raphes pontis. The MRN is located between the posterior end of the superior cerebellar peduncles and the V. Afferents of the motor nucleus. It is one of two nuclei, the other being the dorsal raphe nucleus (DnR), in the midbrain-pons.

In the hippocampus, the mossy fiber pathway consists of unmyelinated axons projecting from granule cells in the dentate gyrus that terminate on modulatory hilar mossy cells and in Cornu Ammonis area 3 (CA3), a region involved in encoding short-term memory. These axons were first described as mossy fibers by Santiago Ramón y Cajal as they displayed varicosities along their lengths that gave them a mossy appearance. The axons that make up the pathway emerge from the basal portions of the granule cells and pass through the hilus of the dentate gyrus before entering the stratum lucidum of CA3. Granule cell synapses tend to be glutamatergic, though immunohistological data has indicated that some synapses contain neuropeptidergic elements including opiate peptides such as dynorphin and enkephalin. There is also evidence for co-localization of both GABAergic and glutamatergic neurotransmitters within mossy fiber terminals. GABAergic and glutamatergic co-localization in mossy fiber boutons has been observed primarily in the developing hippocampus, but in adulthood, evidence suggests that mossy fiber synapses may alternate which neurotransmitter is released through activity-dependent regulation.
The trisynaptic circuit, or trisynaptic loop is a relay of synaptic transmission in the hippocampus. The circuit was initially described by the neuroanatomist Santiago Ramon y Cajal, in the early twentieth century, using the Golgi staining method. After the discovery of the trisynaptic circuit, a series of research has been conducted to determine the mechanisms driving this circuit. Today, research is focused on how this loop interacts with other parts of the brain, and how it influences human physiology and behaviour. For example, it has been shown that disruptions within the trisynaptic circuit lead to behavioural changes in rodent and feline models.

Hippocampus anatomy describes the physical aspects and properties of the hippocampus, a neural structure in the medial temporal lobe of the brain. It has a distinctive, curved shape that has been likened to the sea-horse monster of Greek mythology and the ram's horns of Amun in Egyptian mythology. This general layout holds across the full range of mammalian species, from hedgehog to human, although the details vary. For example, in the rat, the two hippocampi look similar to a pair of bananas, joined at the stems. In primate brains, including humans, the portion of the hippocampus near the base of the temporal lobe is much broader than the part at the top. Due to the three-dimensional curvature of this structure, two-dimensional sections such as shown are commonly seen. Neuroimaging pictures can show a number of different shapes, depending on the angle and location of the cut.

The fascia dentata is the earliest stage of the hippocampal circuit. Its primary input is the perforant path from the superficial layers of entorhinal cortex. Its principal neurons are tiny granule cells which give rise to unmyelinated axons called the mossy fibers which project to the hilus and CA3. The fascia dentata of the rat contains approximately 1,000,000 granule cells. It receives feedback connections from mossy cells in the hilus at distant levels in the septal and temporal directions. The fascia dentata and the hilus together make up the dentate gyrus. As with all regions of the hippocampus, the dentate gyrus also receives GABAergic and cholinergic input from the medial septum and the diagonal band of Broca.

The name granule cell has been used for a number of different types of neurons whose only common feature is that they all have very small cell bodies. Granule cells are found within the granular layer of the cerebellum, the dentate gyrus of the hippocampus, the superficial layer of the dorsal cochlear nucleus, the olfactory bulb, and the cerebral cortex.
An autapse is a chemical or electrical synapse from a neuron onto itself. It can also be described as a synapse formed by the axon of a neuron on its own dendrites, in vivo or in vitro.
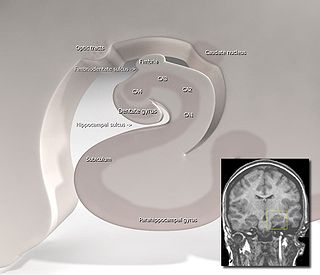
The hippocampus proper refers to the actual structure of the hippocampus which is made up of three regions or subfields. The subfields CA1, CA2, and CA3 use the initials of cornu Ammonis, an earlier name of the hippocampus.
An axo-axonic synapse is a type of synapse, formed by one neuron projecting its axon terminals onto another neuron's axon.













