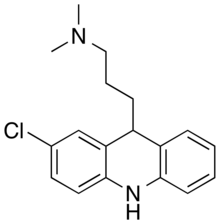
Tacrine is a centrally acting acetylcholinesterase inhibitor and indirect cholinergic agonist (parasympathomimetic). It was the first centrally acting cholinesterase inhibitor approved for the treatment of Alzheimer's disease, and was marketed under the trade name Cognex. Tacrine was first synthesised by Adrien Albert at the University of Sydney in 1949. It also acts as a histamine N-methyltransferase inhibitor.

Mesoridazine(Serentil) is a phenothiazine class drug that is used in the treatment of schizophrenia. It is one of the active metabolites of thioridazine. The drug's name is derived from the methylsulfoxy and piperidine functional groups in its chemical structure.

Levomethorphan (LVM) (INN, BAN) is an opioid analgesic of the morphinan family that has never been marketed. It is the L-stereoisomer of racemethorphan (methorphan). The effects of the two isomers of racemethorphan are quite different, with dextromethorphan (DXM) being an antitussive at low doses and a dissociative hallucinogen at much higher doses. Levomethorphan is about five times stronger than morphine.
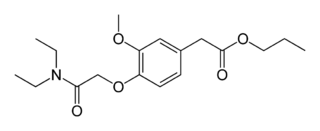
Propanidid is an ultra short-acting phenylacetate general anesthetic. It was originally introduced by Bayer in 1963 but anaphylactic reactions caused it to be withdrawn shortly afterwards.

Iproclozide is an irreversible and selective monoamine oxidase inhibitor (MAOI) of the hydrazine chemical class that was used as an antidepressant, but has since been discontinued. It has been known to cause fulminant hepatitis and there have been at least three reported fatalities due to administration of the drug.

Triclofos is a sedative drug used rarely for treating insomnia.

Pipradrol (Meratran) is a mild central nervous system stimulant that is no longer widely used in most countries due to concerns about its abuse potential. Pipradrol is still used in some European countries and in the United States, albeit rarely.
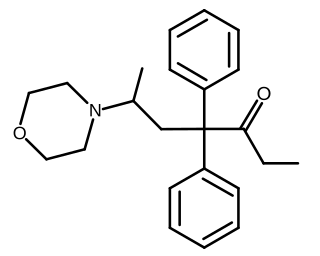
Phenadoxone is an opioid analgesic of the open chain class invented in Germany by Hoechst in 1947. It is one of a handful of useful synthetic analgesics which were used in the United States for various lengths of time in the 20 or so years after the end of the Second World War but which were withdrawn from the market for various or no known reason and which now are mostly in Schedule I of the United States' Controlled Substances Act of 1970, or in Schedule II but not produced or marketed in the US. Others on this list are ketobemidone (Ketogin), dextromoramide, phenazocine, dipipanone, piminodine (Alvodine), propiram (Algeril), anileridine (Leritine) and alphaprodine (Nisentil).

Benzylmorphine (Peronine) is a semi-synthetic opioid narcotic introduced to the international market in 1896 and that of the United States very shortly thereafter. It is much like codeine, containing a benzyl group attached to the morphine molecule just as the methyl group creates codeine and the ethyl group creates ethylmorphine or dionine. It is about 90% as strong as codeine by weight.

Methylpentynol is a tertiary pentynol with hypnotic/sedative and anticonvulsant effects and an exceptionally low therapeutic index. It was discovered by Bayer in 1913 and was used shortly thereafter for the treatment of insomnia, but its use was quickly phased out in response to newer drugs with far more favorable safety profiles.

Norpethidine is a 4-phenylpiperidine derivative that is both a precursor to, and the toxic metabolite of, pethidine (meperidine). It is scheduled by UN Single Convention on Narcotic Drugs. It is a Schedule II Narcotic controlled substance in the United States and has an ACSCN of 9233. The 2014 annual manufacturing quota was 11 grams (0.39 oz).

Pethidinic acid is a 4-phenylpiperidine derivative that is both a metabolite of and a precursor to pethidine (meperidine). It is scheduled by UN Single Convention on Narcotic Drugs. It is a Schedule II Narcotic controlled substance in the United States and has an ACSCN of 9234. The 2014 annual manufacturing quota was 6 grams.

Normorphine is an opiate analogue, the N-demethylated derivative of morphine, that was first described in the 1950s when a large group of N-substituted morphine analogues were characterized for activity. The compound has relatively little opioid activity in its own right, but is a useful intermediate which can be used to produce both opioid antagonists such as nalorphine, and also potent opioid agonists such as N-phenethylnormorphine. with its formation from morphine catalyzed by the liver enzymes CYP3A4 and CYP2C8.

Methyldihydromorphine is a semi-synthetic opioid originally developed in Germany in 1936, controlled under both domestic law and UN conventions because of its possible potential for abuse. Methyldihydromorphine is related to heterocodeine and is not a synonym for dihydrocodeine or dihydroheterocodeine (6-methoxydihydromorphine).

Dixyrazine, also known as dixypazin (oxalate), sold under the brand names Ansiolene, Esocalm, Esucos, Metronal, and Roscal, is a typical antipsychotic of the phenothiazine group described as a neuroleptic and antihistamine. It was first introduced in Germany in 1969. It is used as a neuroleptic, anxiolytic, and antihistamine in doses between 12.5 and 75 mg a day.

Prothipendyl, also known as azapromazine or phrenotropin, is an anxiolytic, antiemetic, and antihistamine of the azaphenothiazine group which is marketed in Europe and is used to treat anxiety and agitation in psychotic syndromes. It differs from promazine only by the replacement of one carbon atom with a nitrogen atom in the tricyclic ring system. Prothipendyl is said to not possess antipsychotic effects, and in accordance, appears to be a weaker dopamine receptor antagonist than other phenothiazines.

Oxymesterone, also known as methandrostenediolone, as well as 4-hydroxy-17α-methyltestosterone or 17α-methylandrost-4-en-4,17β-diol-3-one, is an orally active anabolic-androgenic steroid (AAS). It was known by 1960.

Mefexamide, also known as mefexadyne and mexephenamide, is a central nervous system stimulant that is no longer marketed.

Hydroxyphenamate or oxyfenamate is a sedative and anxiolytic drug of the carbamate class which is no longer marketed in the US. Like other carbamate sedatives, it is chemically related to meprobamate (Miltown). It was introduced to the US market in 1961. The dosage for adults is 200 mg 3 to 4 times daily.

Homofenazine is an antipsychotic drug of the phenothiazines class. It was synthesized by Wilhelm Schuler and colleagues at Degussa. In 1966, it was released in Belgium under the brand name Pasaden. At some point, it was quietly discontinued and is no longer marketed.