Dehydration reactions in organic chemistry resulting in unsaturated bonds
Nitrile formation
Nitriles are often prepared by dehydration of primary amides.
- RC(O)NH2 → RCN + H2O
Ketene formation
Ketene is produced by heating acetic acid and trapping the product: [1]
- CH3CO2H → CH2=C=O + H2O
Alkene formation
Alkenes can be made from alcohols by dehydration. This conversion, among others, is used in converting biomass to liquid fuels. [2] The conversion of ethanol to ethylene is a fundamental example: [3] [4]
- CH3CH2OH → H2C=CH2 + H2O
The reaction is accelerated by acid catalysts such as sulfuric acid and certain zeolites. These reactions often proceed via carbocation intermediates as shown for the dehydration of cyclohexanol. [5]
Some alcohols are prone to dehydration. 3-Hydroxylcarbonyls, called aldols, release water upon standing at room temperature:
- RC(O)CH2CH(OH)R' → RC(O)CH=CHR' + H2O
The reaction is induced by dehydrating reagents. For example, 2-methyl-cyclohexan-1-ol dehydrates to 1-methylcyclohexene in the presence of Martin's sulfurane, which reacts irreversibly with water. [6] [7]
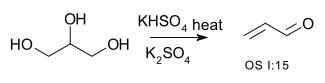
Double dehydration is illustrated by the conversion of glycerol to acrolein: [8] [9]