
The term narcotic originally referred medically to any psychoactive compound with numbing or paralyzing properties. In the United States, it has since become associated with opiates and opioids, commonly morphine and heroin, as well as derivatives of many of the compounds found within raw opium latex. The primary three are morphine, codeine, and thebaine.
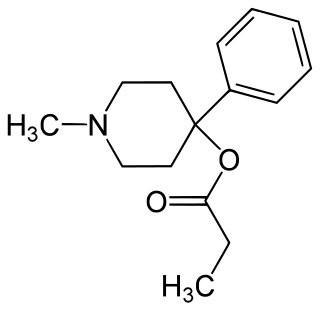
Desmethylprodine or 1-methyl-4-phenyl-4-propionoxypiperidine is an opioid analgesic drug developed in the 1940s by researchers at Hoffmann-La Roche. Desmethylprodine has been labeled by the DEA as a Schedule I drug in the United States. It is an analog of pethidine (meperidine) a Schedule II drug. Chemically, it is a reversed ester of pethidine which has about 70% of the potency of morphine. Unlike its derivative prodine, it does not exhibit optical isomerism. It was reported to have 30 times the activity of pethidine and a greater analgesic effect than morphine in rats, and it was demonstrated to cause central nervous system stimulation in mice.

Pethidine, also known as meperidine and sold under the brand name Demerol among others, is a fully synthetic opioid pain medication of the phenylpiperidine class. Synthesized in 1938 as a potential anticholinergic agent by the German chemist Otto Eisleb, its analgesic properties were first recognized by Otto Schaumann while working for IG Farben, in Germany. Pethidine is the prototype of a large family of analgesics including the pethidine 4-phenylpiperidines, the prodines, bemidones, and others more distant, including diphenoxylate and analogues.

Dipipanone, sold under the brand names of Pipadone and Diconal is a strong opioid analgesic drug, used for acute pain by mouth (PO) for adults. It is often used in instances where morphine is indicated but cannot be used due to the patient being allergic to morphine. In analgesic potency 25 mg dipipanone is approximately equivalent to 10 mg morphine.

Phenoperidine, is an opioid analgesic which is structurally related to pethidine and is used clinically as a general anesthetic.
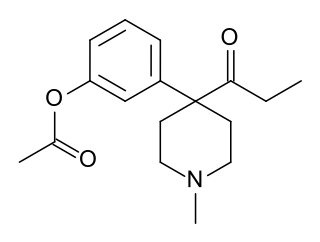
Acetoxyketobemidone (O-Acetylketobemidone) is an opioid analgesic that is an acetylated derivative of ketobemidone. It was developed in the 1950s during research into analogues of pethidine and was assessed by the United Nations Office on Drugs and Crime but was not included on the list of drugs under international control, probably because it was not used in medicine or widely available. Nevertheless, acetoxyketobemidone is controlled as an ester of ketobemidone, which is included in Schedule I of the Single Convention on Narcotic Drugs of 1961.

Allylprodine is an opioid analgesic that is an analog of prodine. It was discovered by Hoffman-La Roche in 1957 during research into the related drug pethidine. Derivatives were tested to prove the theory that phenolic and non-phenolic opioids bind at different sites of the opiate receptor.

Propiram is a partial μ-opioid receptor agonist and weak μ antagonist analgesic from the ampromide family of drugs related to other drugs such as phenampromide and diampromide. It was invented in 1963 in the United Kingdom by Bayer but was not widely marketed, although it saw some limited clinical use, especially in dentistry. Propiram reached Phase III clinical trials in the United States and Canada.

Ethoheptazine is an opioid analgesic from the phenazepane family. It was invented in the 1950s and is a ring expanded analogue of pethidine.

Norpethidine is a 4-phenylpiperidine derivative that is both a precursor to, and the toxic metabolite of, pethidine (meperidine). It is scheduled by UN Single Convention on Narcotic Drugs. It is a Schedule II Narcotic controlled substance in the United States and has an ACSCN of 9233. The 2014 annual manufacturing quota was 11 grams (0.39 oz).
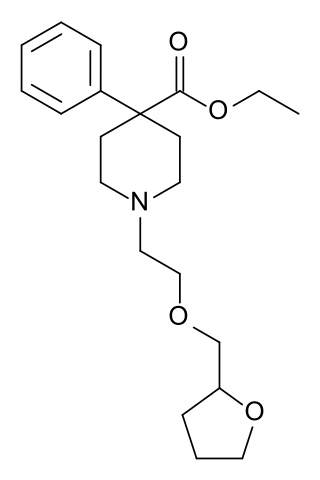
Furethidine is a 4-phenylpiperidine derivative that is related to the clinically used opioid analgesic drug pethidine (meperidine), but with around 25x higher potency. According to another source, Furethidine is 500/30 = 16.7 x the potency of pethidine.

Morpheridine (Morpholinoethylnorpethidine) is a 4-phenylpiperidine derivative that is related to the clinically used opioid analgesic drug pethidine (meperidine). It is a strong analgesic with around 4 times the potency of pethidine, and unlike pethidine, does not cause convulsions, although it produces the standard opioid side effects such as sedation and respiratory depression.

Oxpheneridine is a 4-phenylpiperidine derivative that is related to the opioid analgesic drug pethidine (meperidine).
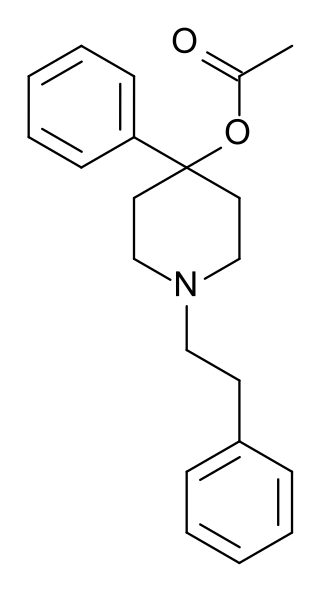
PEPAP (phenethylphenylacetoxypiperidine) is an opioid analgesic that is an analog of desmethylprodine.

Pethidine intermediate A is a four-phenylpiperidine derivative that is a precursor to the opioid analgesic drug pethidine (meperidine). It is not known to have any analgesic activity in its own right, however other derivatives of pethidine with a 4-cyano group in place of the carboxylate ethyl ester have been found to be active, so pethidine intermediate A might also show opioid effects. It is scheduled by UN Single Convention on Narcotic Drugs. It is a Schedule II Narcotic controlled substance in the United States and has an ACSCN of 9232. The 2014 annual manufacturing quota was 6 grammes.
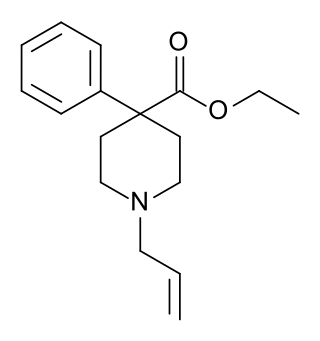
Allylnorpethidine (WIN-7681) is a 4-phenylpiperidine derivative that is related to the opioid analgesic drug pethidine (meperidine).

4-Fluoropethidine is a drug that is a derivative of pethidine (meperidine), which combines pethidine's opioid analgesic effects with increased monoamine reuptake inhibition. It is around 50% less potent than pethidine as an opioid analgesic, but conversely is 50% more potent as a dopamine reuptake inhibitor, with other derivatives such as the 4-iodo and 3,4-dichloro analogues being even more potent dopamine reuptake inhibitors again. However, none of these compounds substitute for cocaine or produce stimulant effects in animals, suggesting that they still act primarily as opioid analgesic drugs in practice. Its action and degree of relation to pethidine means that it may be controlled in those countries which have laws about controlled-substance analogues; it is not itself listed in the Controlled Substances Act 1970.

LS-115509 is an opioid analgesic related to the 4-phenylpiperidine family. It is comparable to drugs such as prodine and pheneridine, but is distinguished by the presence of an ether group and furan ring at the piperidine 4-position, which are not found in other drugs of this class. In animal studies, it has around 2-3x the potency of morphine depending on what assay is used. Like prodine, it has two stereocenters and four possible enantiomers, but the activity of these has not been tested separately.

OPPPP is one of several compounds derived from MPPP, the reversed ester of the opioid analgesic pethidine, which were sold as designer drugs in the 1980s, but have been rarely encountered by law enforcement since the passage of the Federal Analogue Act in 1986. In animal studies it was found to be around 1000× the potency of pethidine, making it several times the potency of fentanyl and with similar hazards of respiratory depression and overdose. It is closely related to numerous compounds made by Janssen et al. for which the structure-activity relationship is well established.
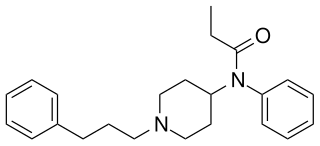
Homofentanyl is an opioid derivative which has been sold as a designer drug. It is a homologue of fentanyl, with similar analgesic and sedative effects but lower potency, around 14× stronger than pethidine.




















