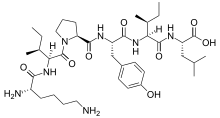
Pro-opiomelanocortin (POMC) is a precursor polypeptide with 241 amino acid residues. POMC is synthesized in corticotrophs of the anterior pituitary from the 267-amino-acid-long polypeptide precursor pre-pro-opiomelanocortin (pre-POMC), by the removal of a 26-amino-acid-long signal peptide sequence during translation. POMC is part of the central melanocortin system.

β-Endorphin (beta-endorphin) is an endogenous opioid neuropeptide and peptide hormone that is produced in certain neurons within the central nervous system and peripheral nervous system. It is one of three endorphins that are produced in humans, the others of which include α-endorphin and γ-endorphin.

Levocabastine (trade name Livostin or Livocab, depending on the region) is a selective second-generation H1 receptor antagonist which was discovered at Janssen Pharmaceutica in 1979. It is used for allergic conjunctivitis.

Neurotensin is a 13 amino acid neuropeptide that is implicated in the regulation of luteinizing hormone and prolactin release and has significant interaction with the dopaminergic system. Neurotensin was first isolated from extracts of bovine hypothalamus based on its ability to cause a visible vasodilation in the exposed cutaneous regions of anesthetized rats.
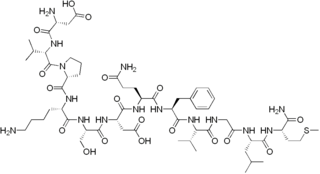
Kassinin is a peptide derived from the Kassina frog. It belongs to tachykinin family of neuropeptides. It is secreted as a defense response, and is involved in neuropeptide signalling.

Met-enkephalin, also known as metenkefalin (INN), sometimes referred to as opioid growth factor (OGF), is a naturally occurring, endogenous opioid peptide that has opioid effects of a relatively short duration. It is one of the two forms of enkephalin, the other being leu-enkephalin. The enkephalins are considered to be the primary endogenous ligands of the δ-opioid receptor, due to their high potency and selectivity for the site over the other endogenous opioids.
Neurotensin receptors are transmembrane receptors that bind the neurotransmitter neurotensin. Two of the receptors encoded by the NTSR1 and NTSR2 genes contain seven transmembrane helices and are G protein coupled. Numerous crystal structures have been reported for the neurotensin receptor 1 (NTS1). The third receptor has a single transmembrane domain and is encoded by the SORT1 gene.

Carboxypeptidase E (CPE), also known as carboxypeptidase H (CPH) and enkephalin convertase, is an enzyme that in humans is encoded by the CPE gene. This enzyme catalyzes the release of C-terminal arginine or lysine residues from polypeptides.
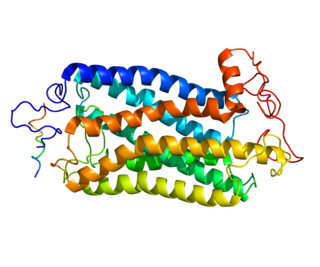
The tachykinin receptor 1 (TACR1) also known as neurokinin 1 receptor (NK1R) or substance P receptor (SPR) is a G protein coupled receptor found in the central nervous system and peripheral nervous system. The endogenous ligand for this receptor is Substance P, although it has some affinity for other tachykinins. The protein is the product of the TACR1 gene.
Neuromedin U is a neuropeptide found in the brain of humans and other mammals, which has a number of diverse functions including contraction of smooth muscle, regulation of blood pressure, pain perception, appetite, bone growth, and hormone release. It was first isolated from the spinal cord in 1985, and named after its ability to cause smooth muscle contraction in the uterus.

N-formyl peptide receptor 3 (FPR3) is a receptor protein that in humans is encoded by the FPR3 gene.

Tachykinin receptor 3, also known as TACR3, is a protein which in humans is encoded by the TACR3 gene.

Neurotensin receptor type 2 is a protein that in humans is encoded by the NTSR2 gene.

Neurotensin receptor type 1 is a protein that in humans is encoded by the NTSR1 gene. For a crystal structure of NTS1, see pdb code 4GRV. In addition, high-resolution crystal structures have been determined in complex with the peptide full agonist NTS8-13, the non-peptide full agonist SRI-9829, the partial agonist RTI-3a, and the antagonists / inverse agonists SR48692 and SR142948A, as well as in the ligand-free apo state., see PDB codes 6YVR (NTSR1-H4X:NTS8–13), 6Z4V (NTSR1-H4bmX:NTS8–13), 6Z8N (NTSR1-H4X:SRI-9829), 6ZA8 (NTSR1-H4X:RTI-3a), 6Z4S (NTSR1-H4bmX:SR48692), 6ZIN (NTSR1-H4X:SR48692), 6Z4Q, and 6Z66.

Rhodopsin-like receptors are a family of proteins that comprise the largest group of G protein-coupled receptors.

Neurolysin, mitochondrial is a protein that in humans is encoded by the NLN gene. It is a 78-kDa enzyme, widely distributed in mammalian tissues and found in various subcellular locations that vary with cell type. Neurolysin exemplifies the ability of neuropeptidases to target various cleavage site sequences by hydrolyzing them in vitro, and metabolism of neurotensin is the most important role of neurolysin in vivo. Neurolysin has also been implicated in pain control, blood pressure regulation, sepsis, reproduction, cancer biology pathogenesis of stroke, and glucose metabolism.
Neuromedin S is a 36-amino acid neuropeptide found in the brain of humans and other mammals. It is produced in the suprachiasmatic nucleus of the hypothalamus and is related to neuromedin U. It is thought to be involved in regulation of circadian rhythm and also has appetite suppressant effects, as well as regulating the release of several other peptide hormones including vasopressin, luteinizing hormone, and oxytocin.

An Oligopeptidase is an enzyme that cleaves peptides but not proteins. This property is due to its structure: the active site of this enzyme is located at the end of a narrow cavity which can only be reached by peptides.
CNMamide (CNMa) is a cyclic neuropeptide identified by computational analysis of Drosophila melanogaster protein sequences and named after its C-terminal ending motif. A gene encoding CNMa was found in most arthropods and comparison among the precursor sequences of several representative species revealed high conservation, particularly in the region of the predicted mature peptide. Two conserved cysteine residues enveloping four amino acids form a disulfide bond and were shown to be important for binding of the peptide to its receptor. Expression of CNMa was confirmed in the larval and adult brain of D. melanogaster but the function of the peptide has not been elucidated yet.

Urotensin II-related peptide (URP) is a cyclic neuropeptide that is found in all vertebrates that have been genome sequenced so far. It has a long lasting hypotensive effect and may also regulate reproduction. It is part of the Urotensin II system and is one of the two endogenous ligands for rats, mice, and possibly humans.